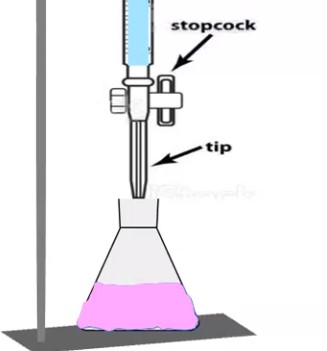
Preparation and Standardization of Potassium Permanganate
Here is a detailed discussion on How to Prepare and Standardization of Potassium Permanganate (1M, 0.1M, 1N, 0.1N) solution by using different reagents in Labs. Preparation and Standardization of 1M Potassium Permanganate Potassium permanganate – M.Wt – 158 Preparation of 1M Solution of Potassium Permanganate: Principle: In volumetric analysis, many reactions involve oxidation and reduction. … Read more