Learn About Standard Operating Procedures for Daily Verification of Weighing balance along with their, Purpose, Scope, Responsibility, Precautions, Procedure, Operation, and Tolerance limit.

1.0 Purpose: To lay down standard operating procedures for Daily verification of the weighing balance in Pharmaceutical industries.
2.0 Scope: This Daily Verification of Weighing balance is applicable for all the weighing balances used in the Pharmaceutical Departments.
3.0 Responsibility: 3.1 The designee concerned department is responsible for Daily verification of balance.
3.2 Head Production/ engineering/ warehouse /Quality Assurance and quality control are responsible for ensuring overall compliance with this SOP.
4.0 Precautions:
4.1 Avoid impact /mechanical shocks to the balance.
4.2 Ensure that the balance platform/ pen is not touching any surface.
4.3 Before an operation, ensure that the balance is clean, dry, and labeled properly.
4.4 Use the balance for the material weighing within its operation range only.
4.5 check the spirit level of the balance for its proper position. Use an external spirit level for balance which is not provided for it.
4.6 Warm-up balance for 5 minutes before starting verification. Keep the weight in the center of the balance to ensure that the standard weight used for verification is within the range and within the due date of certification/ calibration.
4.7 Use calibrated Standard weight for verification of the balance. Consider the certified value of the calibrated standard weight for the calculation of tolerance.
4.8 Reject the standard weight when found defective and replace it with the new certified weight.
4.9 Ensure that there is no weight on the platform of the balance when it is not in use.
Related Topic: Weighing balance Calibration
5.0 Operation of weighing balance:
5.1 Ensure that the balance is connected to an electric point With a cord attached to the balance.
5.2 Check that the spirit level of the balance is in its proper position. If we require, adjust the level using leveling screw, which is provided at the base of the balance.
5.3 Check for zero errors and correct if any by pressing the Zero button.
5.4 If the Zero error is not eliminated, check the reason for the zero error for rectification of the zero error.
5.5 Following remedies should be done to eliminate zero error in case of Daily verification of weighing balance:
- Check the balance using a spirit level.
- Check for mechanical and electronic defects.
- Check the cleanliness of balance.
- Remove the material, which is touching the balance and check for zero error.
5.6 Record the weight displayed and remove the material from the platform.
5.7 Switch off the balance and main power supply at the end of the day.
6.0 Daily verification of weighing balance:
6.1 Verify the balance Daily before the start of the weighing, also verify the balance after a power failure, relocation, and whenever it is required.
6.2 Operate the balance as described in steps 5.1 To 5.7.
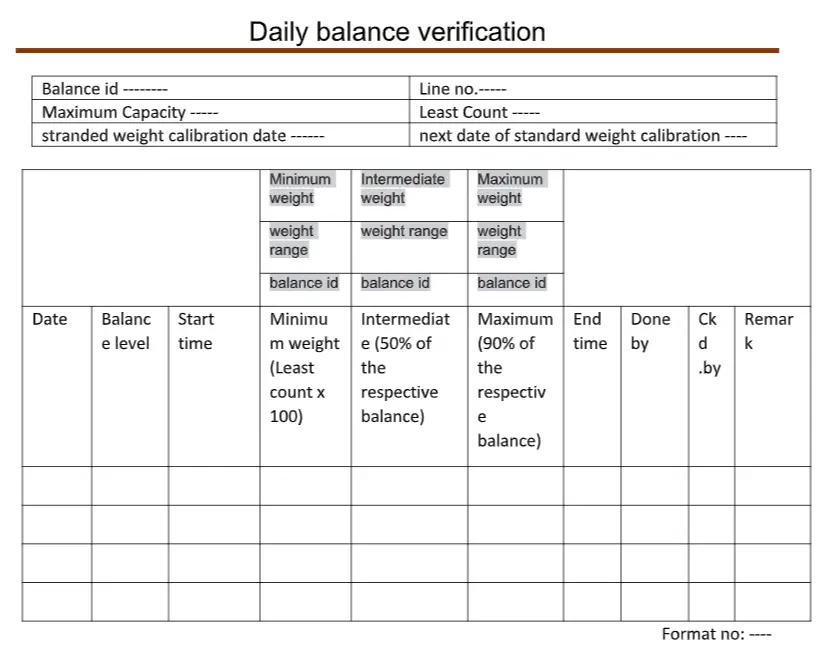
6.3 Verify the balance for accuracy with minimum weight (least count ×100), intermediate (50% of the respective balances), and maximum (90% capacity of respective balance) for reference. Although some companies follow a Maximum 80% capacity of balance.
Note: For a balance having a capacity equal to or more than 500 kg, verify the balances based on the maximum operating range.
6.4 Record the displayed weight in respective verification formats. Fill the verification label as per “Status labeling SOP” Daily signed with a Marker Pen.
6.5 Tolerance: The variation (if any) should be ± least count of the balance or ± 0.2% of the certified value of standard weight used Whenever it is higher. For analytical balance, the variation should be ± least count of the balance or ± 0.1% of the certified value of standard weight used, whichever is higher.
7.0 Abbreviations:
% – Percentage
g – Gram
LC – Least count
NLT – Note Less Than
SOP – Standard Operating procedure
wt. – Weight
Annexure for daily verification of balance:

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].

GOOD SOP for our recoed purpose