Due to the growing demand for GMP and regulatory requirements in the pharmaceutical industry, there is a high chance of deviations occurring. These deviations must be documented, and the manufacturing organization recognizes the need for a standard operating procedure (SOP) for handling such deviations. However, even with good compliance practices in place, there may still be instances where the approved procedures, processes, or systems require temporary modifications or changes.
Definition of Deviation:
The Deviation is any modification or temporary change in any approved procedure, documentation, or specifications.
21CFR 211.100 states that there will be written procedures for production & process control to assure. however, if there is a deviation from written procedures, it will be documented and justified.
Deviation Reporting:
The deviation report is a document that is used only once and can change the documents inside or mentioned in the batch records. It does not permanently change existing specifications, SOPs, or other batch records documents. These documents could be revised according to the document change control procedure if required.
Deviation Flow Chart
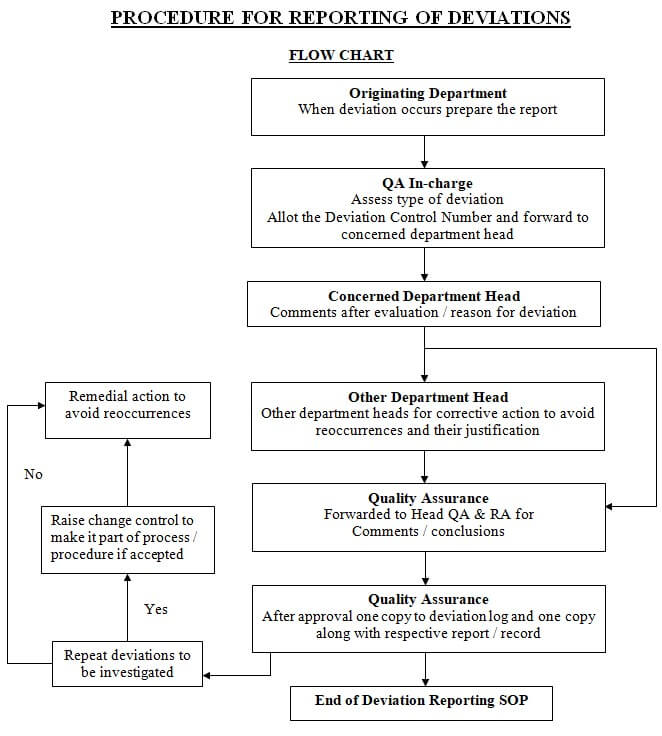
Types of deviations:
Deviation could be process or procedure-related. The following types of deviation can occur in a manufacturing process:
Planned vs. Unplanned Deviations:
Prior to execution, a Planned Deviation is a suggested change to any approved procedure, document, or specification. An example of a planned deviation could be the use of Water for Injection (WFI) instead of purified water to rinse equipment that has been cleaned.
An Unplanned Deviation is an unexpected event that requires a change to any approved procedure, documents, or specifications. it is usually discovered after that fact. An example of an Unplanned deviation is a sudden change in temperature/humidity in the manufacturing area, failed key parameters, or temperature variation outside the specified range. Unplanned Deviation is further classified as minor, major, and critically based on the event and the impact on the product quality, efficacy, and purity.
Related: List of Potential Areas of Deviations
Temporary vs. Permanent Deviation:
The Deviation could be temporary or permanent, depending upon the impact. If the deviation occurs only once, it should be viewed as a one-time occurrence. if the deviation is going to result in permanent changes in approved procedure, document, or specification, then it is a permanent change and will require detailed follow-up corrective action to implement the required change, including employee training.
Lot-specific vs. multiple lot Deviations
The deviation is usually lot-specific for a given drug substance/ product, but can also involve multiple lots.
Product-specific vs. multiple products Deviations:
The Deviation is generally product-specific, but in some cases, it can be involved more than one substance. eg. a sudden failure of the water system, impacting multiple products, which might have used suspect-quality water for equipment cleaning or product manufacture.

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
