Are you looking for the documents required for medical devices? If yes, in this article, you will find a list of those documents, which will be very useful in the future. Let’s discuss each document in detail. Here are the detailed Documents required for Class A medical device as per CDSCO.
Application for grant of Import Licence or Licence to manufacture for sale or for distribution of Class A medical device requires the following documentation under CDSCO:
Site Master File
It shall meet the stated requirements laid down in Schedule 4, Part III, Appendix-1 of Indian Medical Device Rule 2017. The site master file shall have information on the name and address of the firm, including surrounding area information with contact details. Detailed descriptions of the operations will be carried out on your site, including those that are not in your scope of current certification. Plant layout, department-wise employees list with their education and experience details, list of suppliers and service providers, registration of devices outside of India, in-house testing facility, and their equipment list.
The site master file shall have information on key persons with their designation, responsibilities, qualifications, and experience. On-job training procedures and their record. It should also define the health and personal hygiene requirements of persons who are in direct contact with products, keeping in mind device safety and performance, and meeting the requirements of essential principles.
The nature of building construction, plant layout, ventilation system, and classification of clean rooms for products under the scope of certification, storage area for handling hazardous materials if any, and description of the water system used in production and testing. All related procedures and formats shall be referenced in the site master file wherever required.
The site master file shall contain descriptions of the manufacturing and testing equipment. It may be in the form of annexes. It should also include their maintenance plan and calibration plan for measuring and monitoring equipment.
Details of the production process in the form of a process flow chart. Arrangements for the handling of starting materials, packaging materials, bulk, and finished products, including sampling, quarantine, release and storage, reprocessing or rework, handling of rejected materials and products, process validation, and sterilization facility.
Quality assurance for the document control system, internal audit, handling of complaints, field safety corrective action, and contract activities.
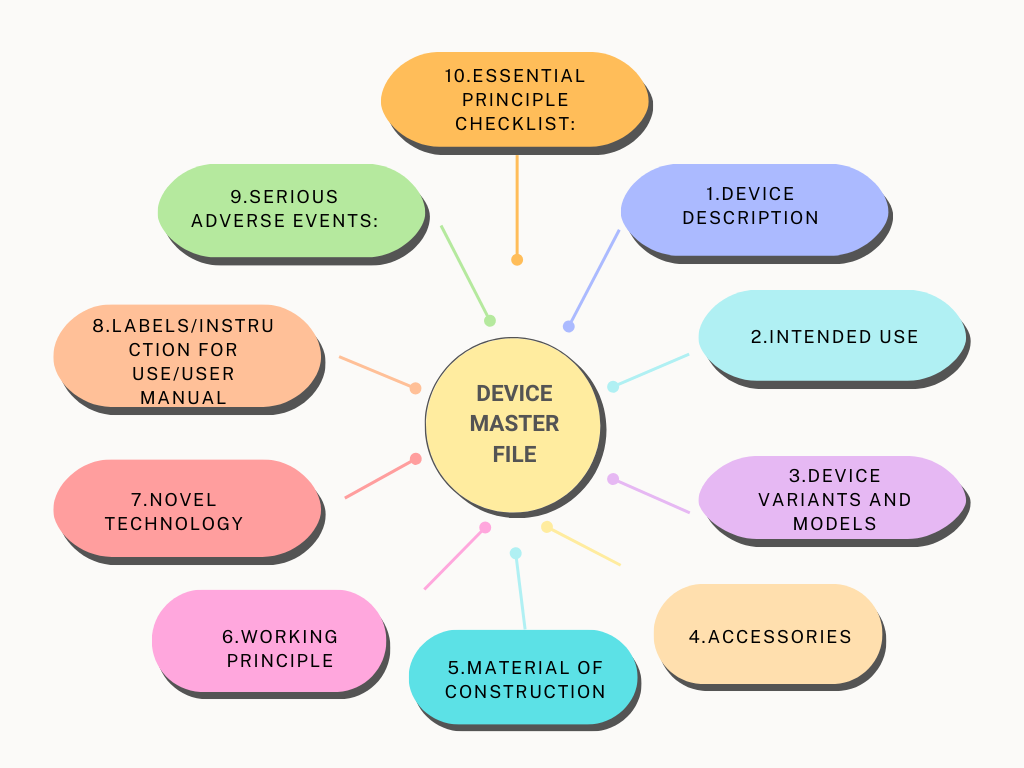
Device Master File:
The device master file for Class A medical devices shall contain the following minimum contents:

Device Description:
Briefly write about your device, including its features.
Intended use:
This section asks you to write the intended use of the device and user specifications for the safe use of the device. Define the patient group for which the device is intended, such as age, gender, site of application, physical condition of the patient, and other requirements that are necessary for the safety and performance of the device.
Related: MDSAP Audit: Concepts, Plan and Requirements
Device variants and models:
This can be in the form of a table as given below:
| Sr. No. | Model Name | Model Number | Brand Name |
Accessories:
This section talks about accessories that are required to achieve the intended purpose of the device. An accessory is a finished device that is intended to support and supplement the performance of one or more parent devices (Source). A parent device is a finished device whose performance is supported, supplemented, and/or augmented by one or more accessories.
Material of construction:
Material used for manufacturing the device must comply with applicable national/international standards. These should meet the requirements of essential principles. Material used for manufacturing the device and its accessories should be listed.
Working Principle:
How the device achieves its intended purpose as per the information supplied by the manufacturer. It may be presented in pictorial form or as a graphical presentation.
Novel technology:
Any new function/feature in your device.
Labels/Instruction for use/User manual:
The device master file must have labels/instructions for use or information on the user manual. It may be referenced.
Serious adverse events:
Add information on adverse events, if any.
Essential Principle Checklist:
Ensure compliance in the form of the essentials principle checklist as per rule no. 6 CDSCO, Notification Number 29/misc/3/2017-DC (179).

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].