A limit test for chloride is done to identify the impurity in the compounds by comparing the test solution against the standard solution. The chloride limits test is only designed to identify and control the impurity present in the compounds.
Limit Test for Chloride Principle
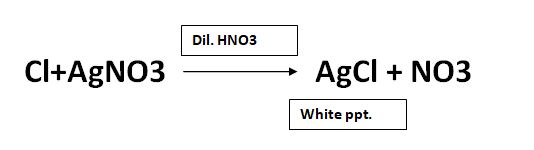
The limit test for chloride is based on the reaction between soluble chloride with silver nitrate in the presence of dilute nitric acid to develop silver chloride, which appears in the solution as solid particles (Opalescence). If the opalescence in the test sample is less than the standard test, it passes the test. If it is more than the standard, it fails the test.
Chemical Required for Test:
- NaCl 0.05845 % W/V
- Distilled water
- Dilute HNO3
- Silver Nitrate 5%
Reason behind Nitric acid usage:
Nitric acid solution has the property to make the solution acidic which will help to make the solution turbid till the end of the procedure.
The reaction of soluble chloride and silver nitrate is as follows:
Procedure for Limit test for chloride:
| Test Sample | Standard Compound |
|---|---|
| Take the test solution and transfer it to a Nessler cylinder. | Take 1 ml of 0.05845 % W/V solution of NaCl in the Nessler cylinder. |
| Take 1 ml of nitric acid in the above solution. | Take 1 ml of nitric acid in the above solution. |
| Dilute the solution with 50 ml distilled water in a Nessler cylinder. | Dilute the solution with 50 ml distilled water in a Nessler cylinder. |
| Take 1ml of silver nitrate solution and add it to the solution. | Take 1ml of silver nitrate solution and add it to the solution. |
| Keep it for 5 minutes to set. | Keep it for 5 minutes to set. |
| After 5 minutes Observe the Opalescence/Turbidity. | After 5 minutes Observe the Opalescence/Turbidity. |
Related: Limit Test for Iron
Observation:
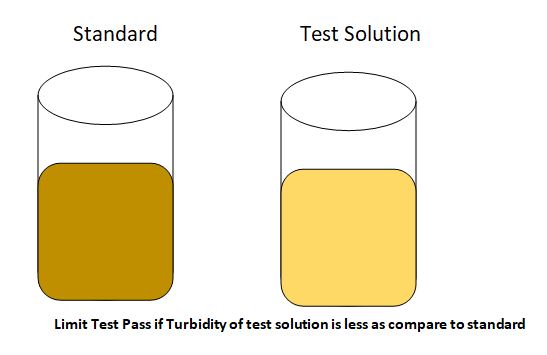
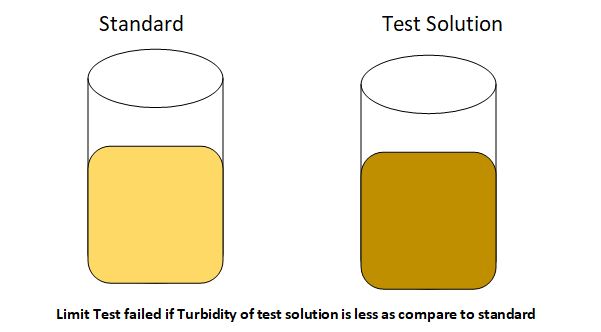
The opalescence produced in the sample solution should not be more than that produced in the reference standard solution. If the opalescence produced in the sample solution is less than that of the standard solution, then, the sample will pass the chloride limit test.
Precaution:
Do not use tap water instead of distilled water, because the presence of chloride in tap water can interfere with the result.
Importance of Chloride limit tests:
- To find out harmful impurities
- To find out avoidable/ unavoidable amounts of impurity
USP limit Test for Chloride:
The USP limit test for chloride involves treating a known volume of the sample with a silver nitrate solution and a potassium chromate solution to produce a yellow precipitate. The concentration of chloride ions in the sample is then determined by measuring the weight of the precipitate. The test has a specified limit of detection, which is the minimum concentration of chloride ions that can be accurately detected using the method.
Modified limit test for chloride and sulphate:
To prepare solutions for the limit test for chlorides and sulphates. A specified amount of the substance is dissolved in distilled water and the volume is made up to 50 ml in a Nessler’s cylinder. Depending upon the nature of the substance, some modifications have to be adopted for the preparation of the solution.
(1) Alkaline substances have to be dissolved in acid to stop effervescence Production and much of the free acid is left in the solution as is prescribed in the test.
(2) Insoluble substances are generally extracted with water and then filtered and the filtrate is used for the test. because the presence of insoluble substances modifies the opalescent colour.
3) Salts of organic acid like sodium benzoate. sodium salicylate. etc. liberate free water-insoluble organic acid during acidification which is filtered off and the filtrate is employed for the test.
(4) Deeply coloured substances have to be decolourized before the test e.g. potassium permanganate is reduced by boiling it with alcohol and the filtrate is used.
Example: Potassium Permanganate (coloured compound): Since potassium permanganate is purple coloured compound. It is decolourised into a colourless solution. So potassium permanganate is boiled with ethanol then filtered a clear colourless solution is obtained which is used for limit test for chlorides.
References:
U.S Pharmacopeia General chapter <221>
Read Also:

Panks Pamyal is a Author and Editor at Pharmaguddu.com. He Worked in Top Pharmaceuticals MNCs in India had a more then 10 years experience in Quality control department. He Delivering most valuable insights and knowledge through this website.
