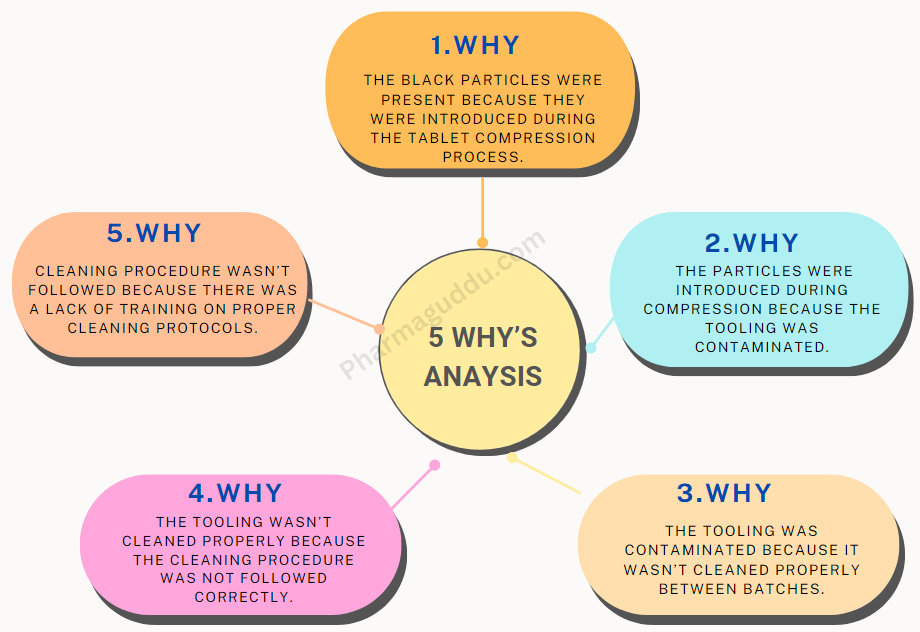
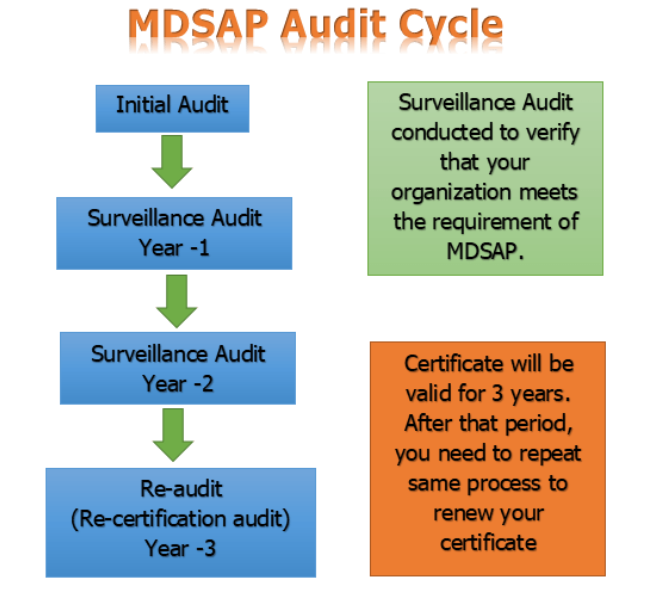
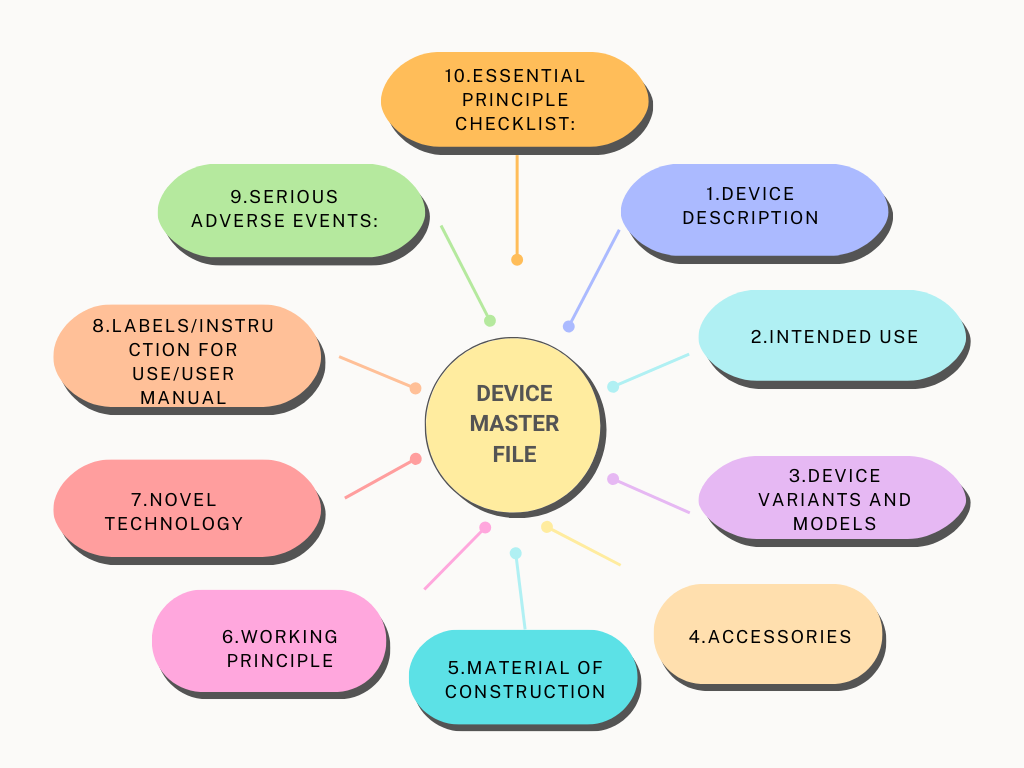
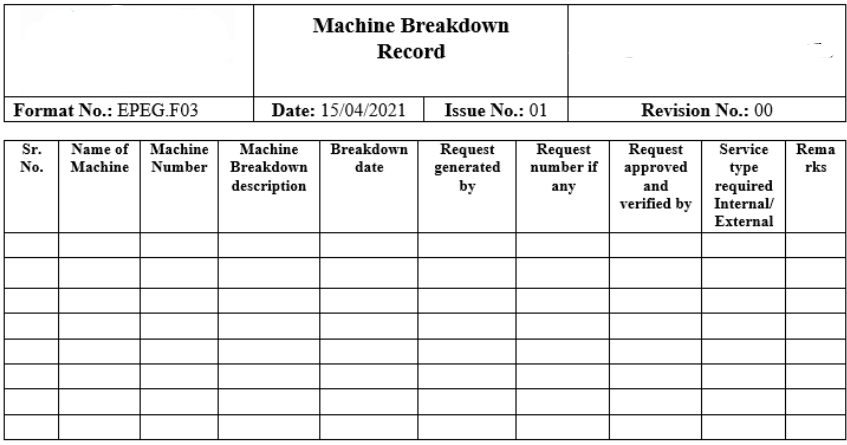
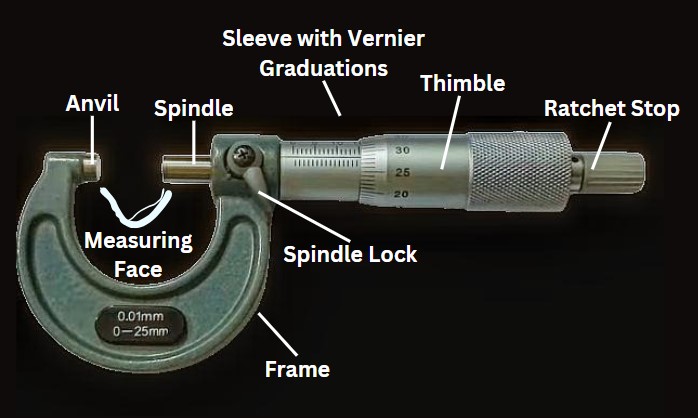
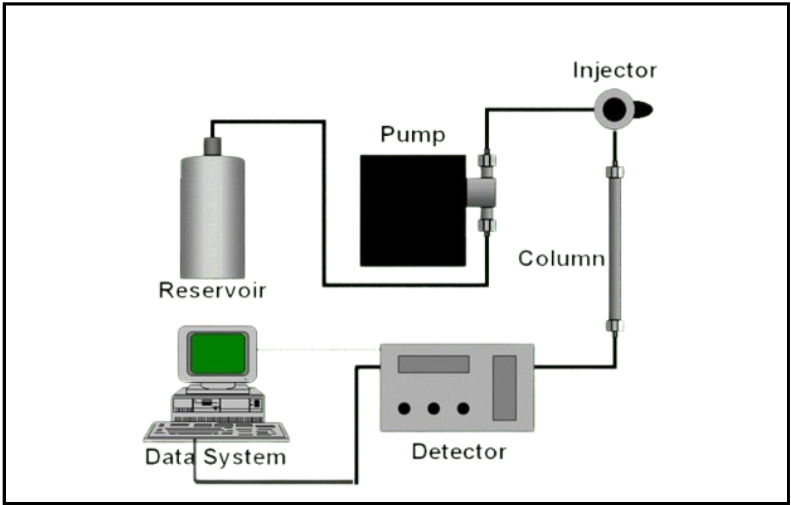
Roles and Responsibilities in Audit
When defining someone’s position in regard to the management of the audit, roles and responsibilities are important of course, but they serve more benefits. It also aids in the productivity of the audit teams. Responsibilities in Audit are certain specific tasks and duties that are allocated to you, in this case, solely. In other words, … Read more