1.0 Objective: To lay down a procedure for monitoring temperature, relative humidity, and differential pressure in the production area.
2.0 Scope: This procedure is applicable to the monitoring of temperature, relative humidity, and differential pressure in the production area.
3.0 Responsibility: Officer, Executive – Production Department
Manager – Production Department
4.0 Procedure:
4.1 Recording of Temperature and relative humidity:
4.1.1 Ensure that the hygrometer is calibrated.
4.1.2 Frequency: At the start of the shift/start of the activity/ or end of the shift.
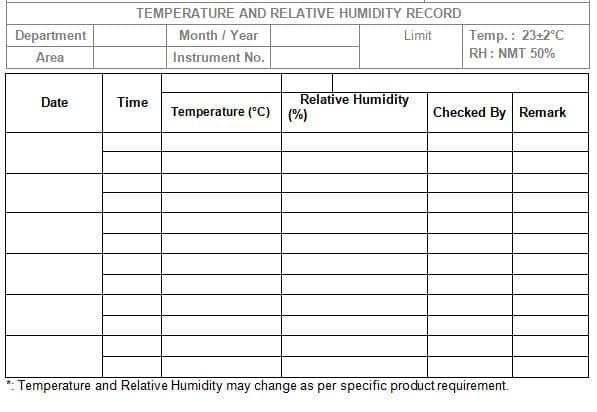
4.1.3 Limit of Temperature:
Operating Limit: 20°C to 25°C.
4.1.4 Limit of Relative Humidity:
Operating Limit: NMT 50%
Note: The limit of temperature and relative humidity may change as per the product
Requirement.
4.1.5 When the temperature exceeds operating limits, the respective responsible officer informs to engineering department by rising intimation, and closes all the in-process material.
For example, Powder in the hopper of the compression machine to be covered with polybags.
4.1.6 On resumption of RH and temperature, the temperature shall be monitored continuously till observations are within limits and then further operations will be carried out.
4.2 Recording of Differential Pressure:
4.2.1 Ensure that the Magnehelic gauge of the concerned area is calibrated.
4.2.2 Ensure the zero error of the Magnehelic gauge by opening the door of the concerned area.
4.2.3 Production Officer shall ensure that all the doors are closed properly before recording the reading.
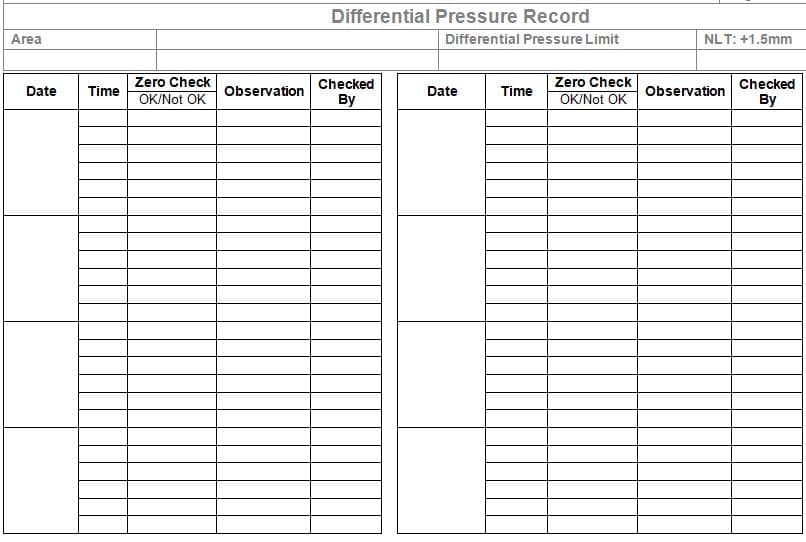
4.2.4 Check the Magnehelic gauge reading and record the same in the format shown in Annexure-II. The reading in the Magnehelic gauge shall be not less than +1.5 mm of the water column.
4.2.5 If there is any deviation in the differential pressure, the same has to be informed immediately to the engineering department as well as to the Quality Assurance department and activity has to be stopped.
4.2.6 The differential pressure recording revealed has to be recorded by the production officer or concerned responsible person.
4.2.7 Frequency: At the start of the shift/start of activity / after product change over / twice in a shift whichever is earlier.
5.0 Abbreviation:
QA: Quality assurance
°C: Degree Celsius
6.0 ANNEXURE(S):
Annexure-I: Temperature and Relative Humidity Record
Annexure-II: Differential Pressure Record.

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
