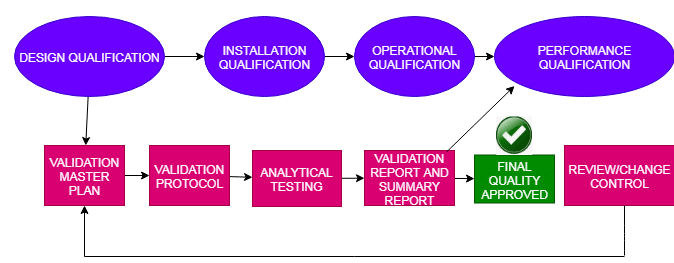
Cleaning Method Validation in Pharmaceutical by FDA
Pharmaceutical products are generally contaminated by microorganisms, cleaning agents, or other materials, But in many cases, it may be due to the repeated use of the same equipment for processing. To avoid this problem FDA introduced the Cleaning method validation process for pharmaceutical industries. The cleaning procedure must be strictly followed to execute the cleaning method. Normally … Read more