Pharmaceutical incompatibility is defined as any unwanted interaction of two or more drugs that results in a significant change either in the physical, chemical, or therapeutic behavior of drugs. The change can also result in an undesirable product which might affect the safety, efficacy, appearance, and stability of the product. Incompatibility can occur during compounding, packaging, dispensing, and storage.
Pharmaceutical incompatibility is categorized into three classes, namely.
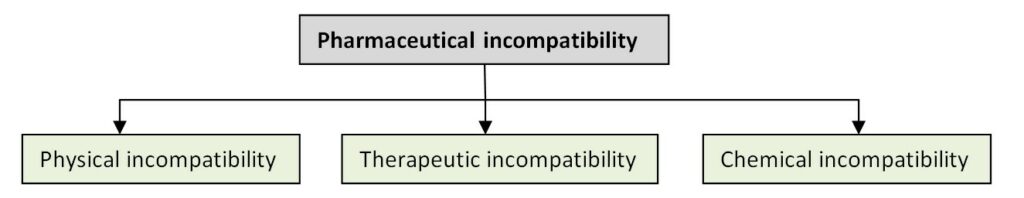
- Physical incompatibility
- Therapeutic incompatibility
- Chemical incompatibility
1. PHYSICAL INCOMPATIBILITY:
Any interaction between two or more components that results in a change in color, odor, taste, viscosity, and morphology is called physical incompatibility. It results in an unacceptable, non-uniform, unpalatable product. Sometimes it affects the metering of dose from a container.
Physical incompatibility can occur due to:
- The insolubility of components in the vehicle
- The immiscibility of two or more liquids
- Liquefaction of solids mixed in a dry state (called eutexia)
Insolubility: Insolubility of a component in a vehicle occurs due to changes in pH, process, or choice of surfactant. Any inadvertent change leads to the precipitation of the drug and changes in its properties. Weak acids in acidic conditions tend to show precipitation while weak bases in alkaline conditions show precipitation. This precipitate formation indicates the insolubility of such drugs at the stated pH. In-diffusible solids such as chalk, zinc oxide, and bentonite show insolubility, therefore wetting agents and thickening agents are incorporated to make a homogeneous formulation. Sometimes potent insoluble drugs are replaced with their equivalent salt derivatives to improve solubility; the example includes atropine sulfate instead of atropine. Sometimes a change of solvent can also prevent insolubility as in the case of chlorophyll tincture where precipitation occurs with dilution with water, therefore dilution with hydroalcoholic solution is required.
Immiscibility: Immiscibility occurs when two or more liquids are incompatible. The manifestation is the appearance of distinct layers in the mixture, emulsion, creams, lotions, and ointments. In the recipe given below:
Rx…
Olive oil -30ml
Water q.s- 120 ml
Make an Emulsion
Olive oil is insoluble in water due to insolubility therefore, a suitable emulsifying agent is added Concentrated hydro alcohols, used as a flavoring agent, form globules when added to aqueous preparations, therefore they are added gradually. The aqueous solution of volatile oil when added to a high concentration of salt solution like potassium citrate in a potassium citrate mixture, oil separates out, therefore, tinctures such as quillaia tincture are added.
Liquefaction: When a low melting solid is mixed with another low melting solid, further lowering of melting point occurs and a liquid state is observed. It is called eutexia or liquefaction. Menthol, thymol, and aspirin form an eutectic mixture. Absorbents like light Kaolin and light magnesium carbonate are added to adsorb the moisture.
Physical incompatibility can be avoided by following the proper order of mixing, changing in a solvent, changing in the form of an active pharmaceutical ingredient, changing the volume of the vehicle used, adding suspending or emulsifying agent, or changing the interfering component.
Rx…
Sulphamethoxazole – 4.0g
Trimethoprim – 0.8g
Sodium carboxy methyl cellulose – 0.5g
Purified water q.s – 100 ml
In the above recipe, sulphamethoxazole and trimethoprim are in-diffusible in water. To make them diffusible, suspending agent sodium (CMC) is used for uniform distribution of solute in the aqueous phase for a sufficiently long time so that dose metering is possible.
2. THERAPEUTIC INCOMPATIBILITY:
Therapeutic incompatibility is defined as any unwanted interaction of two or more drugs leading to a change in the safety and efficacy of drugs or drugs. One of the reasons for therapeutic incompatibility is the prescribed dose. Sometimes over dose or underdose prescribed may result in incompatibility. Also, the biotransformation rate is different in organisms, for example, human beings metabolized meperidine (analgesic) at a rate of 17% per hour but in dogs, it is 70% per hour, and therefore the same “dose cannot be given to humans at a same dose regime. Therapeutic incompatibility is also observed due to genetic differences in the individuals and the differences in species, for example. Black people have a deficiency of glucose-6-phosphate dehydrogenase in red blood cells, therefore, they are susceptible to primaquine and phenacetin, Therefore hemolysis occurs leading to anemia and jaundice.
Doge becomes blind with arise of quinine which is well tolerated by human beings. Digitoxin, a cardiac glycoside, and a phytosteroid, is eliminated through the liver and therefore can be given to patients with erratic kidney function. Digoxin is also prescribed for the same ailment and has a longer-lasting effect but is eliminated through the kidney, therefore cannot be given to such patients.
The mechanisms of Therapeutic incompatibility are divided into two groups:
A. Pharmacokinetics interactions: Pharmacokinetic alterations involve changes in the absorption, distribution, metabolism, and excretion of one drug in the presence of another drug.
B. Pharmacodynamics: Pharmacodynamic interaction involves a change in the action of a drug. It involves synergism, antagonism, altered cellular transport, or effect at the receptor site.
A. Pharmacokinetic Interactions:
Some of the pharmacokinetic interactions involve:
a. Altered GIT absorption: Altered GI absorption of any drug can occur due to changes such as:
- Altered pH: The non-ionized form of a drug is more lipid soluble and more readily absorbed from the GIT than the ionized form. Therefore, these drugs must be given at an interval of 2hours
- Altered intestinal bacterial flora: Antibiotics kill natural intestinal flora thus reducing bacterial inactivation of digitoxin, therefore, a high dose of digitoxin is circulated which increases its toxicity.
- Complexation of chelation
- Drug-induced mucosal damage as caused by NSAIDs such as ibuprofen, aspirin, and acetaminophen
b. Protein binding: Some drugs show their effect once they are bound to proteins in plasma. In case of interaction with other drugs, the most protein-bound drug is displaced by the other drug leaving the free drug circulation in the blood without effect. Sulphonamide, an antibacterial drug has a high affinity for proteins but is displaced by highly bound penicillin, thus Itavigh blood circulation. Another example is an interaction of sulphonamide with warfarin; sulphonamide displaces warfarin leading to bleeding due to the availability of free warfarin.
c. Altered metabolism: The liver is the major site of drug metabolism as most of the enzymes are present there. Some drugs may induce the enzymes responsible for metabolism, for example, Carbamazepine (an antiepileptic drug) increases its own metabolism, and Phenytoin increases the hepatic metabolism of theophylline leading to its decreased level. Also, sonic drugs inhibit the metabolism of other drugs leading to a change in response of the prime drug. Inhibition of the enzyme occurs due to the competition for binding sites, thus the onset of action is retarded. Carbamazepine, an enzyme inducer, is administered with an enzyme inhibitor, verapamil; the effect of the inhibitor predominates. Another example is Erythromycin which inhibits the metabolism of astemizole and terfenadine (antihistaminic agents), therefore increasing their serum concentration which leads to cardiotoxicity.
d. Altered renal excretion: When a drug has a competitive reactivity to the protein that is responsible for the active transport of another drug through renal tubules, alteration in renal excretion occurs, for example, Prohenecid and penicillin when administered together, elevated plasma levels of acidic drug probenecid is observed which leads to toxicity. This is due to an alteration in tubular secretion. Drugs such as penicillin and tetracycline are weak acids and are unionized at acidic pH, thus are reabsorbed into the body. This tubular reabsorption leads to altered drug excretion.
B. Pharmacodynamic Interactions:
Pharmacodynamic alterations do not change the scrum concentration of drugs but a change in response is observed. Such interactions tend to show effects such as:
- Additive effect: It occurs when two or more drugs with the same effect when are combined then the effect is the sum of the individual effects relative to the doses used.
- Synergistic effect: It occurs when two or more drugs, withor_without the same effect are used together to yield a combined effect that is greater than the sum of the individual effects of the drugs.
- Potentiation: It is a particular type of synergistic effect where only one of two drugs exerts the action that is made greater by the presence of the second drug.
- Antagonism: It is an interaction reaction where the opposite effect of synergism is observed when two drugs are combined. The effect is even less than either of the active components added alone. The example includes Protamine sulfate administered as an antidote to the anticoagulant action of heparin.
3. CHEMICAL INCOMPATIBILITY:
Chemical incompatibility is defined as the reaction between two or more substances that results in a change in the chemical properties of the dosage form. Such interactions can occur during compounding and are called immediate incompatibility. It is evident as effervescence, precipitation, or color change. When a chemical incompatibility occurs over a period of time, it is called delayed incompatibility.
Chemical incompatibility can also be categorized as:
A. Tolerated incomparability:
It is a type of chemical incompatibility that is averted by adopting some suitable order of mixing without any alteration made in the active ingredients of the preparation.
B. Adjusted incomparability:
It is a type of chemical incompatibility where the reaction is prevented by the addition or substitution of one of the reacting substances with another component of equal therapeutic value without affecting the medicinal value of the preparation, for example, the substitution of caffeine citrate with caffeine in sodium salicylate and caffeine citrate mixture. A variety of chemical reactions are involved in exhibiting chemical incompatibility. They are listed below:
- Oxidation
- Hydrolysis
- Precipitation
- Gas Formation
- Polymerization
- Isomerization
1. Oxidation: Oxidation is defined as the loss of electrons or gain of oxygen. Sometimes auto-oxidation occurs due to the air present. Certain substances such as metals and some impurities catalyze the oxidation process. Oxidation reaction occurs in solutions faster than in solid dosage forms. Any change in pH affects the drug stability and may accelerate oxidation reactions. The oxidation reaction is evident through color change, odor, and change in viscosity. The use of antioxidants such as vitamin E, vitamin C, and inorganic sulfur compounds is required to prevent oxidation. Using amber color bottles, protection from direct sunlight and storage in a dark place can prevent oxidation.
The examples of recipes that show oxidation are given below:
Example I:
Rx…
Sodium salicylate – 4g
Sodium bicarbonate – 4g
Peppermint water q.s – 60 ml
Make a mixture
In the above recipe, sodium salicylate is oxidized in the presence of sodium bicarbonate leading to color change. The alkali-catalyzed oxidation is prevented by adding 0.1% sodium metabisulphite.
Example 2:
Rx…
Potassium chlorate – 4g
Ferric iodide – 10 ml
Purified water q.s – 60 ml
The above recipe is a mixture where potassium chlorate reacts with ferric iodide to form crystals of iodine upon storage. Therefore, the two compounds are dispensed separately with instructions to mix the two before administration.
Example 3:
Potassuim chlorate – 0.5g
Tannic acid – 0.2g
Sucrose – 0.2g
The above recipe is an example of the incompatibility of oxidizing agent (potassium chlorate) with a reducing agent (tannic acid) leads to the formation of an explosive reaction. Therefore, it is advisable to dispense the components separately, and when required, the mixing should be done very lightly.
2. Hydrolysis: Hydrolysis is a chemical reaction where the presence of water causes a breakdown of compounds. For example, acetylsalicylic acid is hydrolyzed to salicylic acid and acetic acid. This is the reason that aspirin is not available in a solution form. Maintenance of pH, addition of absorbent, and adding stabilizers are some of the approaches to prevent hydrolysis.
3. Precipitation: Most of the drugs are either weak bases or weak acids. therefore they exhibit solubility at a pH where they are mostly ionized. If a solution of a salt of a weakly acidic drug is acidified. the free acid is precipitated. Similarly, precipitation of free base occurs if a solution of a salt of the weakly basic drug is made alkaline.
Example 4:
Rx…
Sodturn salicylate – 4g
Lamon syrup – 20 ml
Purified water – 100 ml
The above recipe is a mixture in which lemon syrup is added as flavoring and it contains citric acid which reduces the pH of the solution ceding to the precipitation of free salicylic acid. In order to overcome this incompatibility, the use of lemon tincture and simple syrup in a ratio of 1:19 is required.
Example 5:
Rx…
Strychnine hydrochloride – 5ml
aromatic spirit of ammonia – 3 ml
Purified water q.s – 100 ml
The above recipe is a mixture of alkaloid salt (Strychnine hydrochloride) which is precipitated in the presence of aromatic spirit of ammonia, an alkaline substance. The precipitate Is diffusible p e, therefore, the proper order of raising is adopted where the drug is first dissolved In half the required quantity of water while the aromatic spirit of ammonia Is dissolved in the remaining portion of water. The two solutions are mixed slowly to avoid precipitate formation. Alkaloids are weak bases. They are almost Insoluble in water but alkaloidal salts are soluble in water. If these salts arc dispensed with alkaline preparations, such as a strong solution of ammonium acetate, an aromatic spirit of ammonia; or a solution of ammonia, the free alkaloid is precipitated.
4. Gas formation: In some recipes, carbon dioxide is evolved due to a chemical reaction between the ingredients of a formulation. Therefore such formulations are packed in an air-tight container and then the mixture is prepared at the time of administration.
Example 6:
Rx…
Sodium bicarbonate – 1.5g
Borax – 1.5g
Phenol – 0.75g
Glycerin – 25 ml
Water to 100ml
The above recipe is an example of a spray where borax, (sodium tetra borate decahydrate (Na2B4O7.10H2O) decomposes in the presence of glycerin to form sodium metaborate and boric acid. Boric acid then reacts with glycerin to form glyceryl boric acid. Glyceryl boric acid reacts with bicarbonate and carbon-dioxide gas is evolved. Since the precipitates are diffusible types, the reacting components are added to one portion of the solvent and to the second portion of the liquid; the other reacting substances are added. Finally, the two solutions are added with stirring. This alteration in the method of mixing is called Method A. Method B is used for in-diffusible precipitates. Here, the vehicle is divided into two equal portions as in the case of Method A, and to one part, the reacting substance is dissolved. To the other part, a suitable amount of suspending agent such as tragacanth (2g per 100m1 of the formulation) is added with trituration to form mucilage. Then the other reacting substance is added with stirring to form a homogeneous and stable product.
Example 7:
Rx…
Ferric chloride solution – 2ml
Sosdium salicylate – 3g
Water q.s – 90ml
In the above recipe, ferric salt reacts with sodium salicylate to liberate in-diffusible precipitates of ferric salicylate therefore method B is followed for precipitate forming interactions.
5. Polymerization: In polymerization, small repeating units called monomers are bonded to form a long-chain polymer.
6. Isomerization: Isomerization is the conversion of a drug to its isomer which has identical molecular formula but a different arrangement of atoms. Optical isomerization is a conversion of an optical active drug into a less active, for example, L-adrenaline is converted to d-adrenaline by a change in pH or temperature. L-adrenaline is more therapeutically active than d-adrenaline, though both have the same physical properties but different arrangements of atoms. Geometric isomerization is where a molecule exhibits cis or trans configuration. Cis configuration indicates the arrangement of atoms in the same direction while trans configuration shows groups in opposite directions. Incompatibility also occurs due to adjuvants added to the formulation. Some of the incompatibilities are listed below:
- Flavoring agent: Glycyrrhiza extraction contains sodium, potassium salts of glycyrrhizinic acid. When such a flavoring is added to acid formulations, black sediment is formed and the flavor is also lost. Therefore, such flavorings are used for basic preparations.
- Colorants: Amaranth is a soluble edible dye that is anionic. When it is added to cationic dyes such as methylene blue, salt formation occurs leading to the formation of Insoluble precipitates and loss of color.
- Preservatives: emulsions with triethanolamine soap are in-compatible with salts of cations, double decomposition leads to polyvalent soap which inverts the emulsion.
- Suspending agents: Negatively charged suspending agents when are added to a positively charged drug, floc formation occurs but when an excess of suspending agent is added, charge reversal occurs leading to the formation of Deflocculated suspension.
- Emulsifying agent: The solubility of emulgent determines the type of emulsion formed. Thus the addition of substances that change the solubility cause phase inversion, for example, o/w emulsion with soap as emulsifying agent changes to w/o emulsion when calcium chloride is added.
Some other examples of chemical incompatibility include:
- Crystal violet is purple in color but changes to green-yellow on acidification. Phenolphthalein is colorless in acidic conditions but becomes pink in alkaline conditions.
- The addition of potassium iodide to ferric chloride solution leads to the liberation of free iodides which is not required therefore, alkali citrate or alkali tartrate prevents the liberation of free iodide, thus to dispense this recipe, potassium citrate is added to ferric chloride solution and then potassium iodide is added. The formulation is used in Goiter.
- A recipe containing sodium salicylate, quinine sulfate, and dilute sulphuric acid shows the formation of in-diffusible precipitates. Here, dilute sulphuric acid which is added to dissolve quinine reacts with sodium salicylate to form insoluble salicylic acid. Therefore, it’s a true incompatibility and cannot be dispensed as such.
- In a recipe containing mercurous chloride, potassium bromide, and sucrose, moisture leads to incompatibility. Mercurous chloride reacts with potassium bromide to form mercuric bromide and liberation of free mercury occurs.
- A recipe containing penicillin G and syrup of cherry is incompatible because penicillin is affected by acidic conditions created by the syrup cheery, therefore, in-diffusible penicilloic acid is formed which is an inactive product.
- Oxidizing chemicals such as potassium chlorate, potassium permanganate, and chromic acid should not be brought into contact with organic oxidizable matter as leads to explosion. Therefore, minimal rubbing is required during preparation.
- Morphine, an alkaloid, is used as a painkiller in ear drops. The recipe also contains tannic acid which is used as an astringent to block discharge from ears. A precipitate formation occurs due to the incompatibility of morphine with tannic acid leading to the formation of morphine tannate.
Frequently Asked Pharmaceutical Incompatibility Questions
Ans: Pharmaceutical incompatibility occurs when drugs interact and modify their properties, affecting their safety and efficacy. It can happen during the compounding, packing, and storage processes.
Ans: Physical (color and odor changes), therapeutic (safety and efficacy changes), and chemical (dosage form changes) incompatibility are the three types.
Ans: Physical incompatibility can be avoided by changing the method of mixing, changing the solvent, and using agents such as emulsifiers.
Ans: Therapeutic incompatibility can be caused by dose variation, genetic differences, and species-specific responses.
Ans: Chemical incompatibility generates reactions that alter the properties of drugs, resulting in rapid or delayed effects. It can be managed with mixing orders and additives.