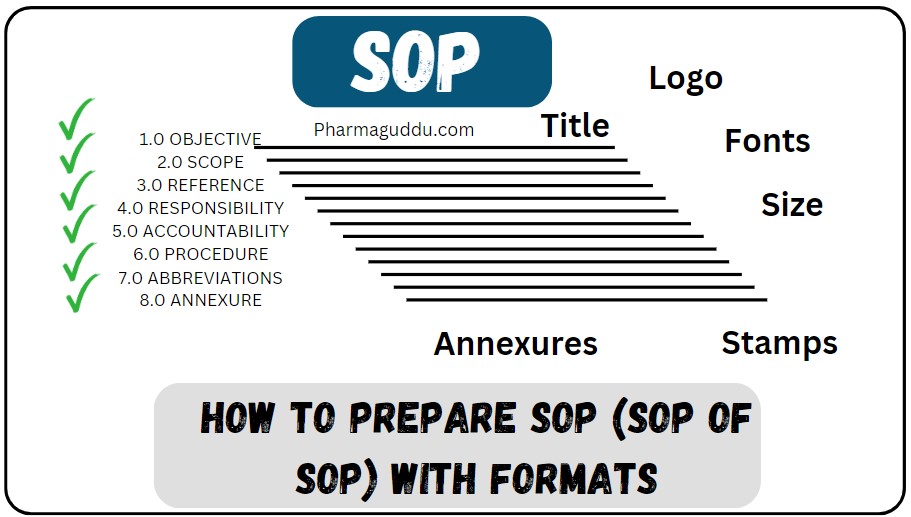
SOP for Cleaning and Operating of Digital Timer
Purpose: To establish a standard operating procedure for cleaning and operating of Digital Timer. Scope: This standard operating procedure is applicable for cleaning and operating of Digital Timer. Responsibility: Accountability: Procedure: Instrument Introduction: Cleaning: Operation of instrument: Time/ Calendar Display Mode: Time and Calendar Setting: Alarm Setting: Stop -Watch Mode: Battery Replacement: Precautions: Maintenance: Calibration: … Read more