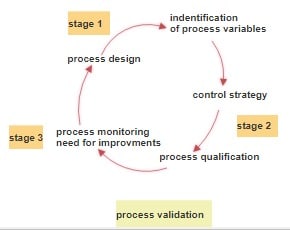
Validation of Fumigation in Clean Room Areas in Pharmaceutical Industries
An uncontaminated environment is essential in pharmaceutical manufacture. Clean rooms created with strict ISO guidelines are engineered with the intent of reducing particulate and microbial contamination in drug manufacturing processes. However, microbial contamination may still be present owing to inadequate cooling, cleaning regimens, or even both. These controlled environments must comply with regulations which means … Read more