When the process of moving technology including (Chemicals, Production/ Manufacturing, Control, and drug product-related documents) from one place to another, so we can make the same good stuff every time, that’s called technology transfer. Below is the full Checklist of Technology Transfer For Sending the Plant.
| Project identification No. | — |
| Name of Product | — |
| Technology sending Coordinator | — |
| Technology Receiving Coordinator (Proposed Site) | — |
Technology Transfer Checklist For Sending Plant
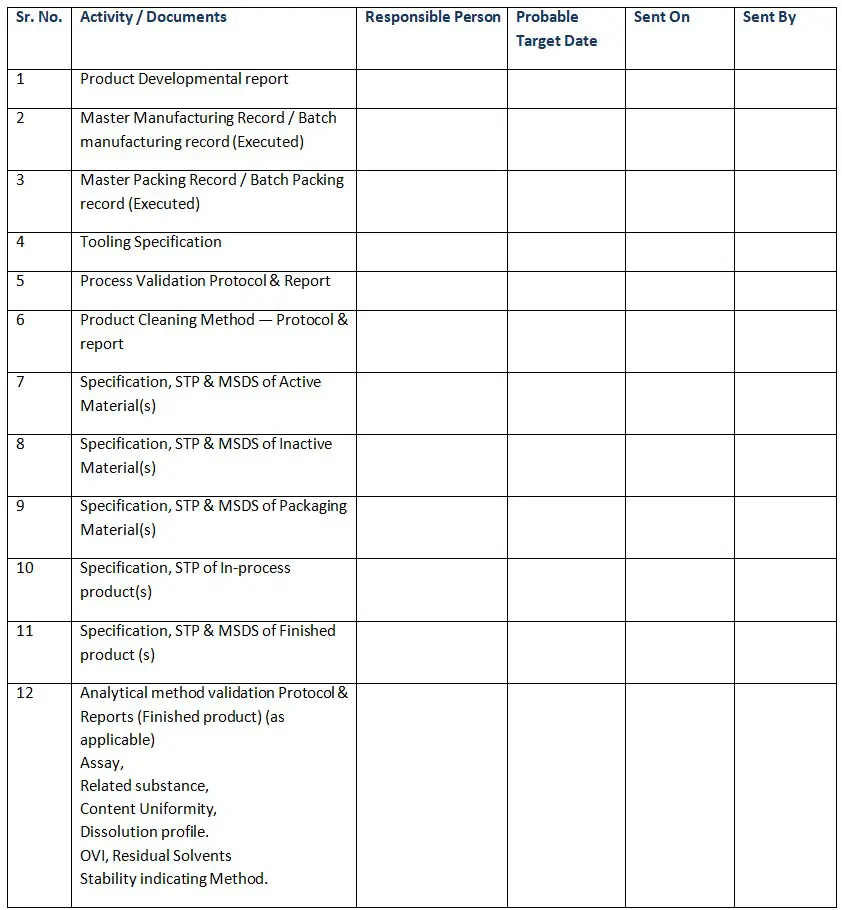
- Product Developmental report
- Master Manufacturing Record / Batch manufacturing record (Executed)
- Master Packing Record / Batch Packing record (Executed)
- Tooling Specification
- Process Validation Protocol & Report
- Product Cleaning Method — Protocol & report
- Specification, STP & MSDS of Active Material(s)
- Specification, STP & MSDS of Inactive Material(s)
- Specification, STP & MSDS of Packaging Material(s)
- Specification, STP of In-process product(s)
- Specification, STP & MSDS of Finished product (s)
- Analytical method validation Protocol & Reports (Finished product) (as applicable)
✔Assay
✔Related substance
✔Content Uniformity
✔Dissolution profile
✔OVI, Residual Solvents
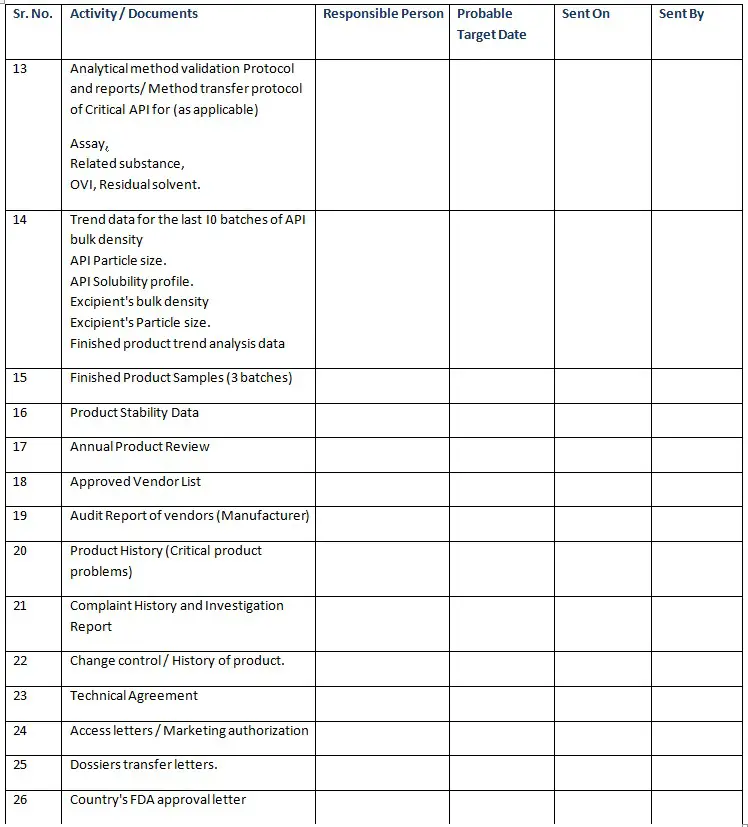
✔Stability indicating Method. - Analytical method validation Protocol and reports/ Method transfer protocol of Critical API for (as applicable ).
✔Assay
✔Related substance
✔OVI, Residual solvent. - Trend data for the last I0 batches of API bulk density
✔API Particle size.
✔API Solubility profile.
✔Excipient’s bulk density
✔Excipient’s Particle size.
✔Finished product trend analysis data - Finished Product Samples (3 batches)
- Product Stability Data
- Annual Product Review
- Approved Vendor List
- Audit Report of vendors (Manufacturer)
- Product History (Critical product problems)
- Complaint History and Investigation Report
- Change control / History of product.
- Technical Agreement
- Access letters / Marketing authorization
- Dossiers transfer letters.
- Country’s FDA approval letter
Related: Example of Product Investigation and Root Cause Analysis and its Report Preparation
Technology Transfer Checklist For Sending Plant (Annexure):
Related: Audit Check List for Quality Assurance Pharmaceuticals