1.0 Objective: To lay down a procedure for the management of sieves/ screens / FBD bowl mesh.
2.0 Scope: The procedure is applicable to check the inventory and integrity of sieves/ screens / FBD bowl mesh in the production department.
3.0 Responsibility:
Officer, Executive – Production Department
Manager – Production Department
4.0 Definitions:
NA
5.0 Procedure:
5.1 For Inventory:
5.1.1 On intimation of receipt from stores, collect all the sieve/ screen / FBD finger bags / RMG filter bags from the packing material de-dusting area.
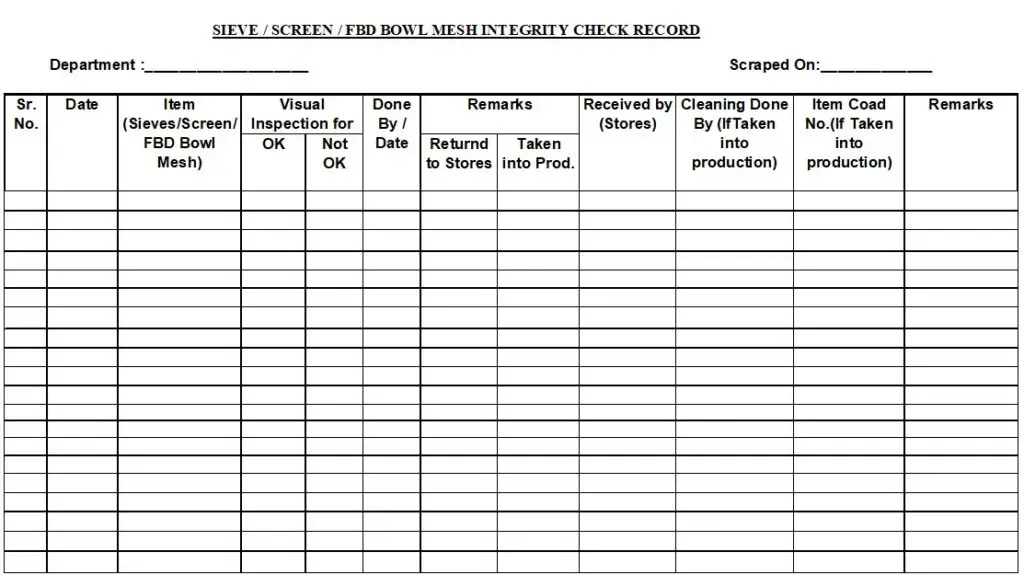
5.1.2 Check the integrity of individual sieve/ screen / FBD finger bags / RMG filter bags against a light source and record the observations in annexure-I.
5.1.3 Remark OK for pass sieve/screen / FBD finger bags / RMG filter bags and Not OK for sieve/screen / FBD finger bags / RMG filter bags which fail the integrity test. Return back the defective sieve/screen / FBD finger bags / RMG filter bags to stores.
5.1.4 Allot code no. as per SOP on “Procedure for Coding Accessories” to the pass sieve/screen / FBD finger bags / RMG filter bags and transfer them to the washroom for cleaning.
Clean the sieve/screen by following SOP No. :
Note: For new sieves/screens received, the sieve/screen shall be cleaned as per SOP “cleaning procedure for all containers, Bins and Sieves” before taking them to the inventory.
5.2 For Integrity:
5.2.1 For sieves:
5.2.1.1 Check the integrity of the sieve and Teflon seal before and after use by holding the sieves against a light source.
5.2.1.2 Scrap the sieve in case of any damage to the sieve.
5.2.2 For screens:
5.2.2.1 Visually check the integrity of the screen before and after use by holding the screen against a light source.
5.2.2.2 Scrap the screen in case of any damage to the screen.
5.2.3 Note:
5.2.3.1 Integrity check of sieve/screen / FBD Bowl Mesh has to be done whenever new sieve/screen are received and recorded in Annexure-I.
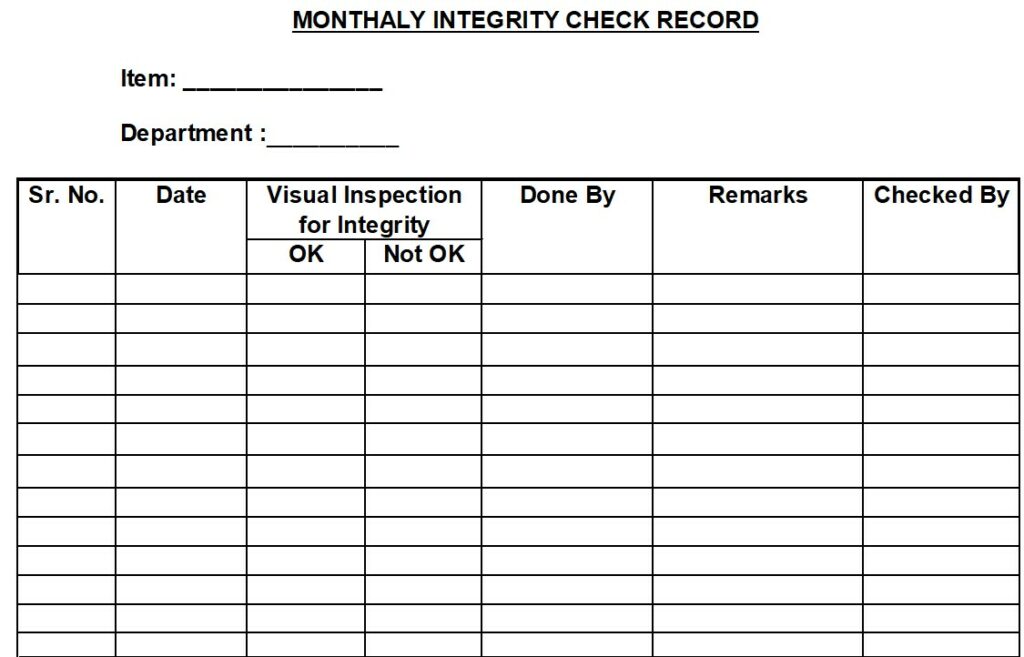
5.2.3.2 Integrity check of sieve/screen / FBD Bowl Mesh has to be done in the first week of every month and record the observations in Annexure-II.
5.2.3.3 If the sieve/screen is found damaged during/after use, Inform to department head and QA.
5.2.3.4 The in-process material shall be kept aside and investigation shall be carried out. Based on the investigation, an action plan is to be prepared.
5.2.3.5 Send the damaged sieve/screen to the engineering department to de-shape before scrapping the sieve/screen.
6.0 Abbreviations:
BMR: Batch Manufacturing Record.
FBD: Fluidized Bed Dryer
SOP: Standard Operating Procedure.
QA: Quality Assurance
7.0 References:
NA
8.0 Annexures:
Annexure –I: Sieve / Screen / FBD Bowl Integrity Check Record.
Annexure –II: Monthly Integrity Check Record.

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
