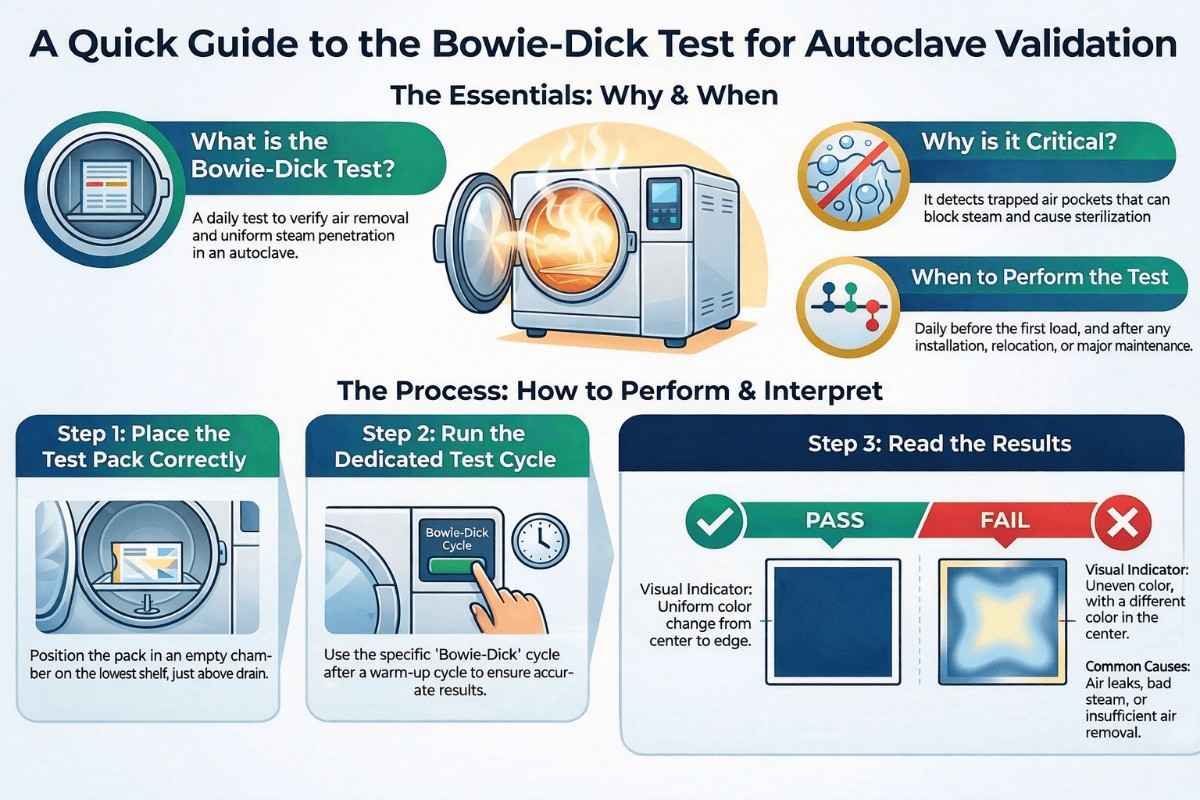
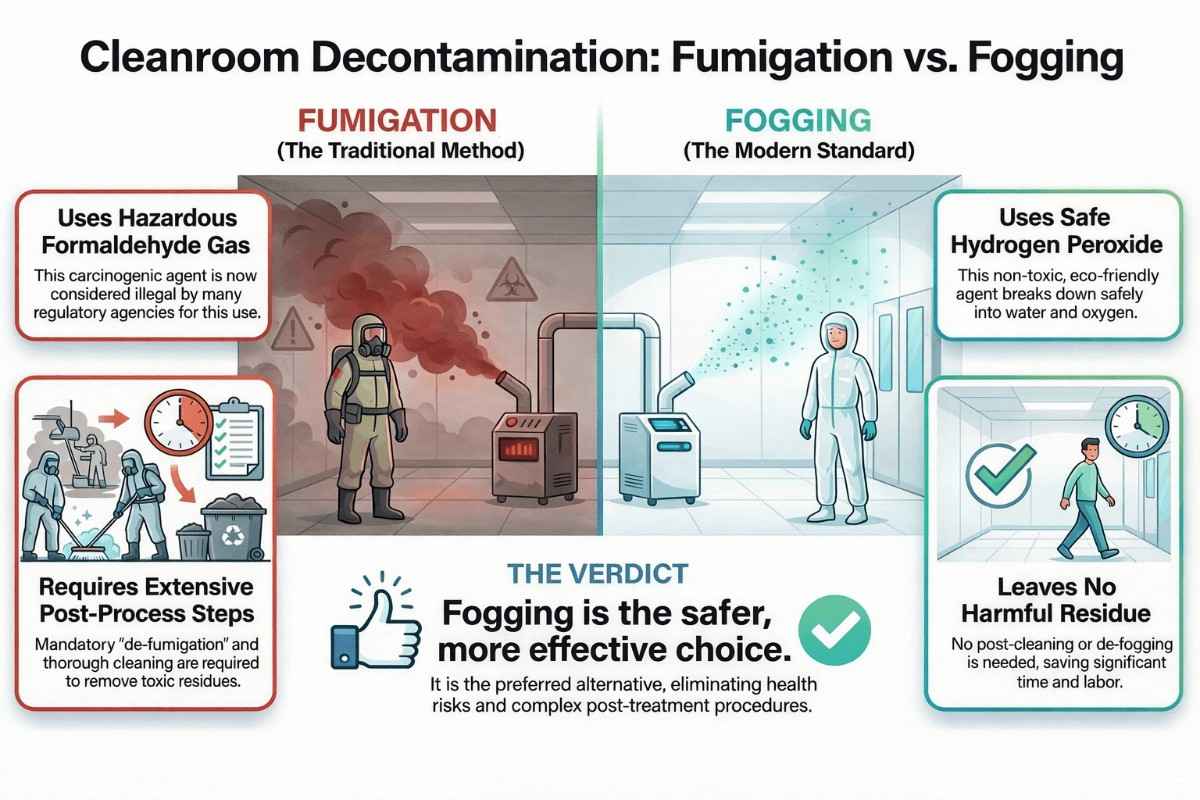
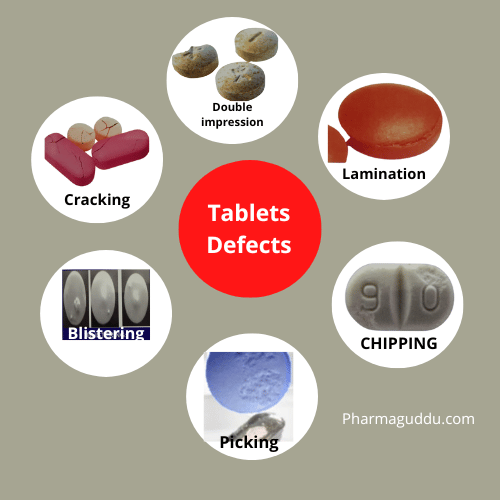
Compression Machine and Tooling, Types, Kit and Cleaning Process
Tablet presses or Compression machines are mechanical devices that compress granulated powders into uniform tablets for pharmaceuticals and other solid-dosage products. These machines force material between matching upper and lower punches seated in a die cavity to fuse it into a solid form. In operation, the lower punch first lowers into the die to create … Read more