1.0 Objective: To lay down a procedure for status labeling in the production department.
2.0 Scope: This procedure is applicable to the Status Labeling of equipment, cleaning and disinfectant aids, lubricating aids, in-process materials, and excess packing materials in the production areas.
3.0 Responsibility: Officer, Executive – Production Department
Manager – Production Department
QA Officer – In the process

4.0 Procedure for Status Labeling:
4.1 Ensure that all equipment, in-process material, finished product, and containers are labeled at all times. No unlabeled equipment, in-process material, finished product, etc., will be taken/used for processing.
4.2 Ensure that all equipment, and containers, are labeled before the start of the activity.
4.3 Ensure that all raw materials / in-process materials / finished products when stored in HDPE /SS containers lined with double polythene bags shall bear a label.
4.4 Ensure that all the bags stored in a container shall be individually labeled.
4.5 Ensure that all the containers are tared without lid before taking for the charging of material.
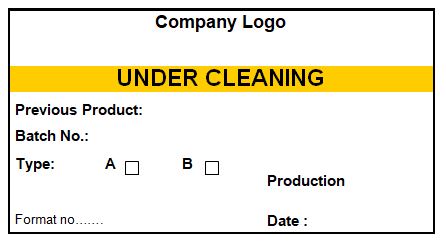
4.7 Ensure that all equipment is labeled with an appropriate status label as per the specimen label given in annexure-I.
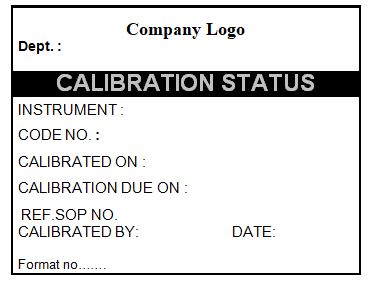
4.8 Ensure that all test equipment is labeled with an appropriate calibration and verification status label as per the specimen label given in annexure-I.
4.9 All cleaning and disinfectant aids, food-grade lubricants, and Isopropyl Alcohol are to be labeled with an appropriate status label as per the specimen given in annexure-I.
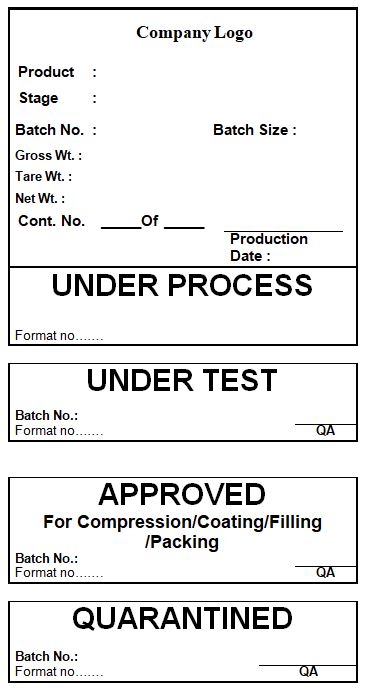
4.10 Ensure that all in-process materials are labeled with an appropriate status label as per the specimen given in annexure-I.
4.11 Ensure that all the process areas bear a status label(board) as per annexure –I.
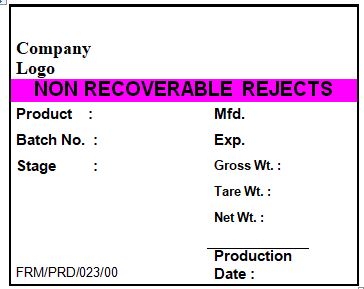
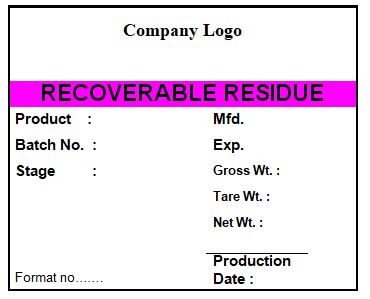
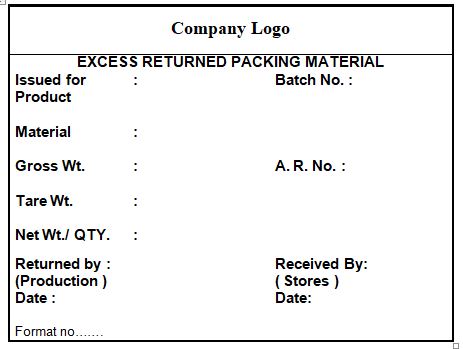
4.12 Ensure that all excess packing materials, which need to be returned to stores are labeled as per the specimen given in annexure-I.
4.13 Ensure that all the punch sets are labeled on storage in their respective cabinet.
4.14 Ensure that all Raw material dispensing tags are with the BMR after verification.
4.15 Ensure that all bulk in-process materials (compressed/ coated tablets) bear appropriate Q.A. status labels.
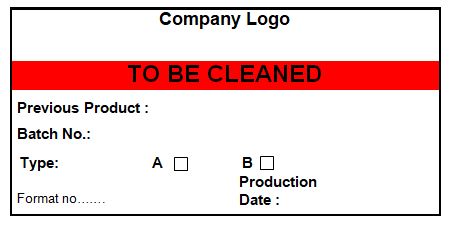
4.16 Ensure that all equipment after cleaning bears a status label as per the specimen label shown in annexure-I, after product changeover (Type B cleaning), the cleaning tags of equipment are to be attached to the BMR/BPR of the next product.
5.0 ABBREVIATION(S):
BMR: Batch Manufacturing Record.
BPR: Batch Packing Record
QC: Quality Control
SS: Stainless steel
Related SOP: SOP on design, print, issuance, and control of logbooks
Annexure-1



Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
