Hold time is the period during which materials ( Machine Parts) may be held under specified conditions or time and remain within the stipulated requirements. At all phases of production Hold time study on machine change parts to be done on a given frequency to ensure the quality of products.
Manufacturers must ensure that the products they create are safe, effective, and of the appropriate quality for their intended application. Products should be constantly made to the quality standards required by the marketing authorization and appropriate for their intended application. Pharmaceutical products should be manufactured using validated methods, according to the systems. Manufacturing procedures should be demonstrated to be capable of consistently producing pharmaceutical products of the specified quality and specifications.
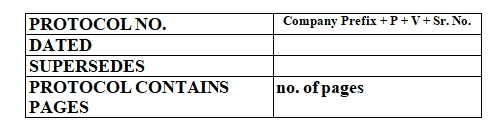
Hold Time Study Of Machine Parts Protocol Must Includes:
Location: Department Name/ Block Name
Line: Specify the line Name
Hold Time Study Of Machine Parts Protocol Contents:
1.0 Protocol Approval
2.0 Unit Operation
3.0 Objective
4.0 Site of study
5.0 Team
6.0 Sop for Operation
7.0 Control
8.0 Acceptance criteria
9.0 Type of Validation
10.0 Frequency
11.0 Experimental Details
12.0 Sample to be Collected
1.0 Protocol approval:
2.0 UNIT OPERATION: Holding period of the sterilized Machine parts of Powder filling machines.
3.0 OBJECTIVE: To ensure that the sterilized machine parts remain sterile during storage and usage period (3 days or 72 Hrs).
4.0 SITE OF STUDY: Department Name (Injection), Company Name, and Site Location.
5.0 TEAM: Representative from:
- Production
- Quality Assurance
- Quality Control
- Engineering
- (Individual to be named in the report)
6.0 SOP FOR OPERATION:
- Cleaning of machine parts as per given SOP.
- Sterilization of the Machine parts as per given SOP.
- Storage of Machine parts as per given SOP:
7.0 CONTROL:
- The rubber stopper should be prepared as per SOP No:
- Sterilized the Machine parts in Autoclave as per the loading pattern described in SOP No:
- Transfer the sterile Machine parts to the LAF.
- Sterilized machine parts are to be stored under LAF.
8.0 ACCEPTANCE CRITERIA:
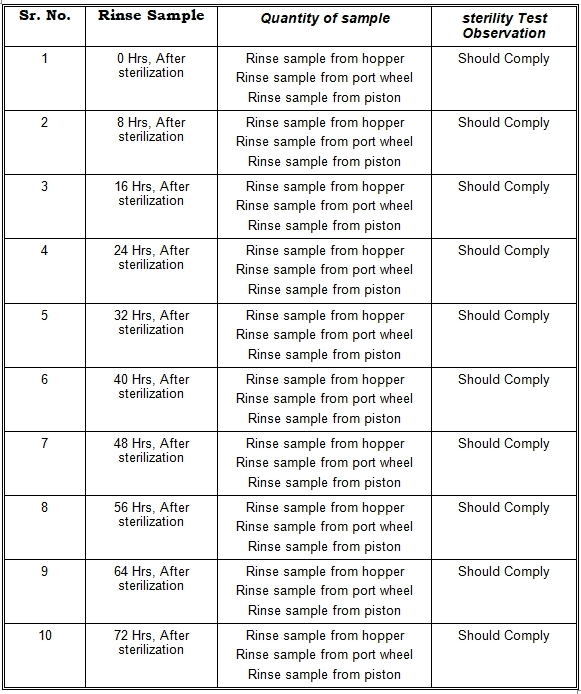
Sterilized Machine parts should pass a sterility test during the storage period of 3 days or 72 Hrs.
9.0 TYPE OF VALIDATION: Prospective validation.
10.0 FREQUENCY: Once in the year for concurrent validation.
11.0 EXPERIMENTAL DETAILS: i) The Machine parts used for the validation should be
sterilized as given SOP.
II ) Transfer of Machine parts should follow as per given SOP.
III) Storage of Machine parts should be done Under LAF started before ½ hours
IV) Differential pressure of the Magnehelic pressure gauge should be within the limit.
Related Post: SOP on Storage and Hold time study for Products
12.0 SAMPLE TO BE COLLECTED:
The sample should be collected from the SS container under LAF:

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
