1.0 Objective: To lay down a procedure for the return of excess packing material in production department to stores.
2.0 Scope: This SOP is applicable for the return of excess packing material in production department to stores.
3.0 Responsibility:
Officer, Executive – Production Department
Manager – Production Department
4.0 Definitions:
NA
5.0 Procedure:
5.1 After completion of the packing activity, Reconcile the primary/ secondary packing material in the prescribed format given in the respective BMR as per SOP “Reconciliation & Destruction of Packing materials“.
5.2 Clean the inner core and outer surface of each roll of PVC, PVC / PVdC, and Aluminum Foil by using a lint-free dry cloth.
5.3 Wrap each roll of printed aluminum foil, PVC, PVC/ PVdC, and secondary packing material (carton, label, shipper, and leaflet) in the polythene bag and label it as ‘EXCESS RETURNED PACKING MATERIAL”.
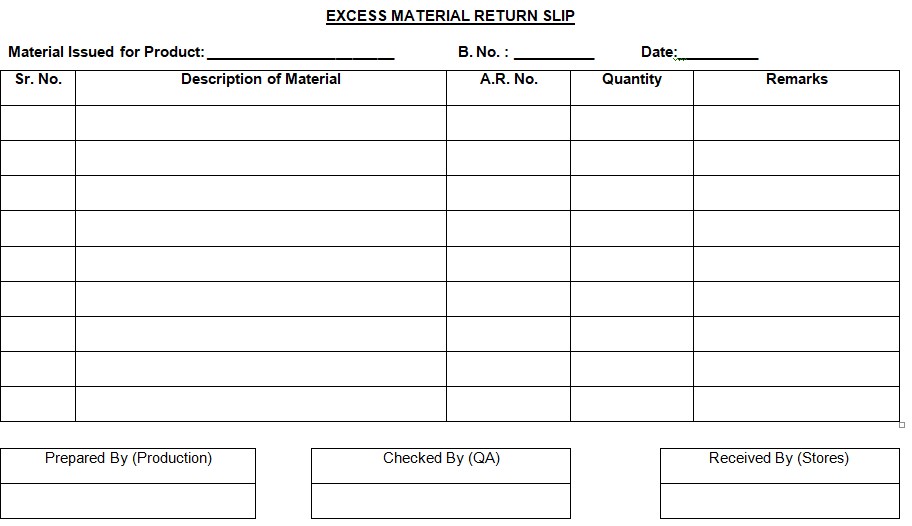
5.4 Record the quantity of unused aluminum foil and PVC, PVC/PVdC film in the ‘EXCESS MATERIAL RETURN SLIP’ (refer to Annexure- 1) and get it verified by the Q.A. officer.
5.5 Record the details in the ‘EXCESS RETURNED PACKING MATERIA’ label and get it verified by Q.A. Officer.
5.6 Destroy all rejected and overprinted secondary packing material (i.e. cartons, labels, shipper, leaflets, polythene bags, inner/outer boxes).
5.7 In case of a change in pack size or plan, the entire dispensed packing material is to be returned to the stores on ‘EXCESS MATERIAL RETURN SLIP’.
5.8 If material is dispensed on a campaign basis (maximum 3 batches), Excess material return slip can be given at the end of campaigning after completion of the last batch i.e. end of 3rd batch.
6.0 Abbreviations:
BMR: Batch Manufacturing Record.
PVC: Poly Vinyl Chloride
PVdC: Poly Vinyled-ene Chloride
Q.A.: Quality Assurance
SOP: Standard Operating Procedure
7.0 References:
SOP: Reconciliation & destruction of packing materials
8.0 Annexures:
Annexure–1: Excess material return slip

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
