1.0 Purpose: To lay down the procedure for Receiving and Transfer the FGTN to Warehouse.
2.0 Aim This Standard Operating Procedure is applicable to the packing Department in Pharmaceutical.
3.0 Responsibility: 3.1 Trained operators/ line coordinators shall be responsible for receiving and transferring the material.
3.2 Officer/Executives of production shall be responsible for checking the activity.
3.3 IPQA in charge shall be responsible for compliance as per SOP.
3.4 Head QA/designee shall be responsible for the authorization of the SOP.
4.0 Procedure for Receiving and Transfer the FGTN to Warehouse:
4.1 For receiving the semi-finished goods from store:
4.1.1 Before receiving the material check the status label on the material. Every material should have a status label as per the “Status labeling” SOP.
4.1.2 Receive the material through static pass Box and check the quantity physically and compared it with the quantity mentioned in the requisition.
4.1.3 Clean the trolley and SS pallets
4.1.4 Check the cleanliness of the cage/trolley and the crates in which material is to be received or transferred.
4.1.5 Transfer the material room
4.2 For storage of material
4.2.1 Store the material in room.
4.2.2 Don’t remove the status label until it is consumed.
4.2.3 Receive & store the material in advance with proper checking of quantity & status before the use of that material.
4.2.4 Ensure the temperature of room is within limit for temperature monitoring and recorded in the logbook.
4.3 For Transfer of finished goods to Warehouse
4.3.1 Check the quantity of finished goods to be transferred to W.H.
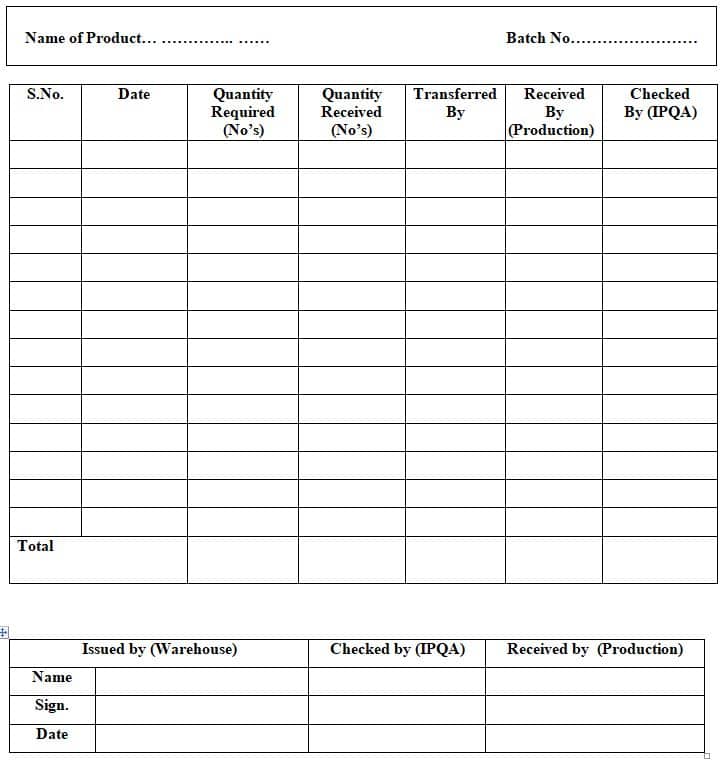
4.3.2 Fill the Annexure No – 2 i.e. FGTN format as per the detail mentioned in it.
4.3.3 The FGTN format should be cross-checked by the IPQA person.
4.3.4 The numbering of FGTN should be started from 001 with the respective year. For every year the number starts from 001.
e.g. S.No/YY, i.e. 001/23
Where S.No: Serial Number, YY: Year (last two digits of the year)
4.3.5 Transfer the sealed and packed shippers to the W.H. storage area through a static pass Box along with FGTN.
4.3.6 After the receiving of finished goods and FGTN by the W.H. store in charge. Attach duplicate copy with BPR.
Related: Receiving, handling, and storage of primary and secondary packaging material
5.0 Abbreviations:
HOD: Head of the Department
ID No. : Identification number
SOP: Standard Operating Procedure
BPR: Batch Processing Record
FGTN: Finished Goods Transfer Note
SFGRN: Semi-Finished Goods Receiving Note
WH: Warehouse
Annexure-1
Annexure-2


Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
