Voltammetry is a time-dependent potential applied to an electrochemical cell, and the current flowing through the cell is measured as a function of that potential. A curve plot of current vs applied potential is called a voltammogram.
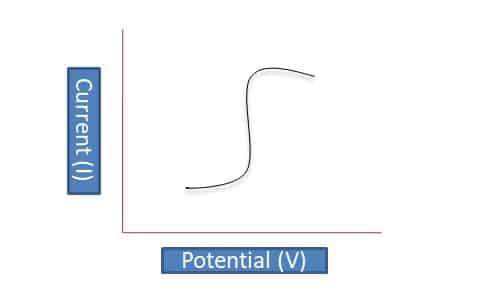
In simple words voltammetry is used to find out the analyte present in the solution by using current as the potential varied. to find out this graph is plotted between current and potential.
The electrolytic cell or Electrochemical cell is used for the electrochemical process. an electrochemical cell contains three electrodes:
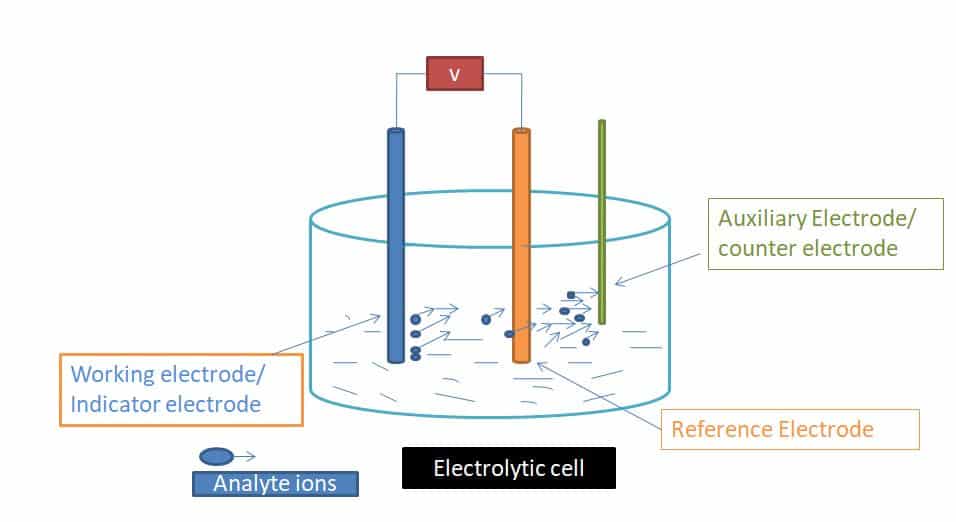
- Working electrodes
- Reference electrodes
- Auxillary electrodes
When an analyte is oxidized or reduced at the working electrode, a current passes electrons through the external electric circuitry to the auxiliary electrode, reference electrodes have known potential so there is no need to change the potential at the reference electrode. so to measure the potential of working electrodes we use auxiliary electrodes.
At the working electrode Reducing an analyte requires a source of electrons, generating a current that flows from the auxiliary electrode to the cathode or working electrode. A current produced by redox reactions at the working and auxiliary electrodes is known as a faradaic current in either condition.
So during the process, as potential varies current also varies as shown in the s-shape graph above.
Cyclic voltammetry:
cyclic voltammetry is different from the voltammetry in sense of using electrodes. the working electrode is made of glassy carbon whereas the reference electrode of Ag/Agcl and auxiliary electrode are made of the platinum disc. The advantage of cyclic voltammetry over the other methods easy to know the final result because of the large potential range.
Polarography:
While using the working electrode dropping mercury electrode (DME) is called Polarography.
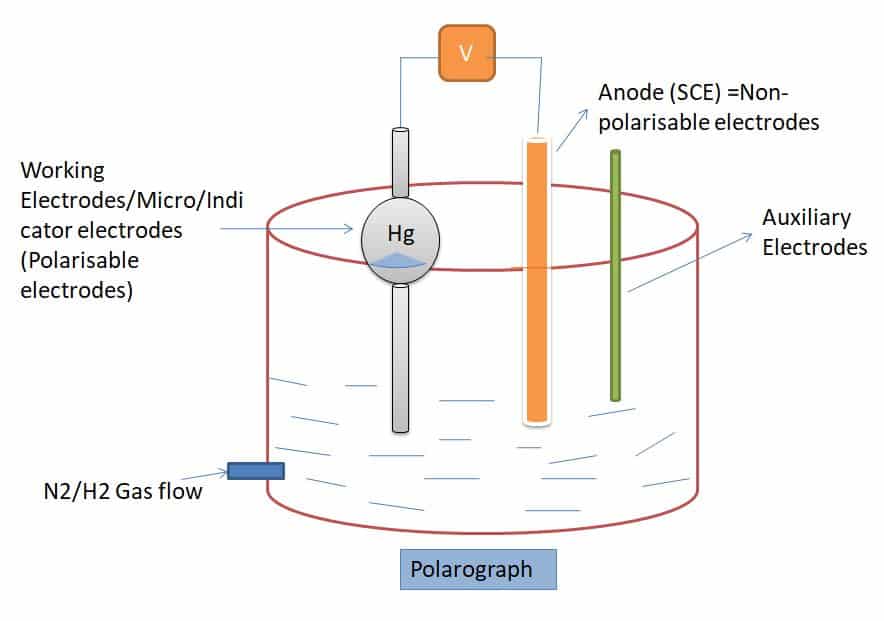
- Hg is filled inside of the Dropping mercuric electrode
- Hg Drop falls inside the solution ( Generally drop cycle taken is 2 to 7 sec.)
- Analyte ions reduce at the cathode (Hg drop)
- Current flow between the working electrode and auxillary electrode while migration of ions.
- Inside the beaker cd(Hg) amalgam is present due to the reduction of cd analyte at the cathode and mix with Hg while drops.
- The potential range is generally taken between (-1.8 to +0.4)
The apparatus for the majority of voltammetric and amperometric methods consists of a working microelectrode, a reference electrode, an auxiliary or counter electrode, an electronic apparatus to control the voltage and voltage sweep, and a computer or recorder to save data.
The dropping mercury electrode (DME), where pure mercury flows through a thin capillary either due to gravitational pull or by applied pressure, was the first microelectrode to be utilized. The typical drop duration is a few seconds. The benefits of this electrode include:
The surface area is tiny and continually refilled to inhibit the formation of electrolytic products;
Mercury has a high potential for hydrogen ion formation, which allows the reduction of other species. Other electrodes used are static or hanging mercury drop electrodes, where the drop is dislodged at a particular time and size, and solid microelectrodes, such as platinum and glassy carbon, which may be interwoven into a rotating disc electrode. Dissolved oxygen in the sample solution must be removed, since oxygen may be reduced in two steps, giving waves that overlap with the sample.
O²+ 2H+ + 2e- H202, E 1/2 = -0.05 V
H202 + 2H+ + 2e- = H20 E1/2 = -0.9 V
Typically, to achieve this during the experiment, oxygen-free nitrogen is passed through the sample solution. Maxima on the waves are due to surface effects and may be suppressed by adding a small amount of surface-active agents, such as gelatin or Triton-X 100.
The reaction that occurs is:
Cu²++H2O= Cu(s) + ½02, + 2H+
Upon the deposit of the copper, oxygen is evolved at the anode and the cathode. This is the basis for electrogravimetry, where the copper is completely deposited from the solution, and the increase in the weight of the cathode is determined, Either a controllable potential or a controlled current can be used to conduct the analysis.
These techniques depend on three elements for ion transport to the electrodes:
Diffusion, Convection or Stirring, and Conduction. The effects of conduction of the ion that reacts at the electrode are minimized by using a concentration of supporting electrolyte such as KCl about 50-times higher than that of the analyte. Stirring and convection are minimized. Three zones can be seen on the resulting polarography curve.
- If a potential difference is applied across a cell and no reaction occurs, only the residual current will flow.
- A reducible ion, such as Cd2+, will move to the mercury cathode that is dropping if one is present. If the applied potential exceeds its decomposition potential, ED will be reduced to the metal. dissolves in the mercury:
Hg- Cd²+2e- = Cd (Hg) As the cadmium plates out, the layer around the electrode is depleted and more cadmium ions must diffuse in from outside through the diffusion layer of thickness d. This will result in the flow of a current, I, whose strength is determined by the gradient of concentration between the surface and the bulk solution. Eventually, the limiting diffusion current is reached and the surface concentration drops to zero:
Id= constant (c(bulk))/d = kS (c(bulk)).
The constant. kS depends on the number of electrons transferred and the diffusion coefficient of the ion in the solution. and the characteristics of the cathode. - If the potential is rise further, the current does not increase unless other reducible ions are present in the solution.
FAQs
Hg(1) because Hg oxidizes to Hg(1) at above +0.4V
A large excess of supporting electrolytes eliminates the migration current.
Dropping mercury electrode is a working electrode
Because it reduces the flow motion of mercury drop
Because of its reproducible and smooth surface, it also enhances sensitivity.
Working electrodes, Reference electrodes, and Auxillary electrodes

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
