Learn about Standard Operating Procedure on FBD Bag including their, Purpose, Scope, responsibility, Procedure for Indenting, Receiving, cleaning, storage, Issuance and Coding of FBD Bag.
1.0 Purpose: To lay down Standard Operating Procedure for Indenting, Receiving, Issuance, Coding, Usage, Cleaning, and Destruction of Fluidized-bed Dryer Bag.
2.0 Scope: This SOP applies to all the FBD Bags used in the Tablet Department at the plant.

3.0 Responsibility:
3.1 Officer Production is responsible for the implementation of this SOP.
3.2 Head Production is responsible for ensuring overall compliance with this SOP.
4.0 Procedure for Indenting of FBD Bag
4.1 Prepare a Purchase Requisition mentioning the Number of Fingers, Diameter in Inches, Material Of Construction, and Quantity.
4.2 Mention the requirement of Material of Construction Certificate from the Manufacturer.
4.3 Get the Purchase Requisition Approved by the Head of Production and Site Head.
4.4 Handover the Approved Purchase Requisition Purchases Department.
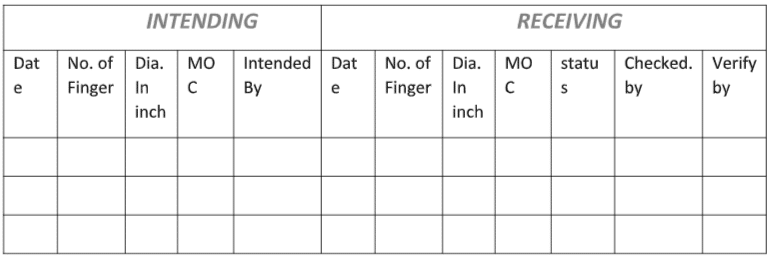
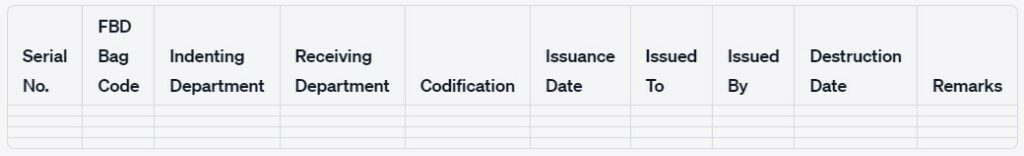
4.5 Record the details of Indenting as per Annexure-II “Indenting, Receiving, Codification, Issuance, and Destruction Record of FBD Bag.”
4.1.0 Receiving of FBD Bag
4.1.1 Receive the FBD Bag from Engineering Store.
4.1.2 Transfer the Pack carrying the FBD Bag to Buffer Room of P.M. Receiving Area of the Warehouse Department.
4.1.3 Clean the External Surface of the Pack with Dry Lint Free Duster.
4.1.4 Transfer the FBD Bag to the Tool Room through Dispensed Primary PM Store.
4.1.5 Check the Physical Condition of FBD Bag.
4.1.6 Check that MOC Certificate from the Manufacturer.
4.1.7 Check that the quantity of FBD Bag received is as per Purchase Order raised.
4.1.8 Check that the Number of Fingers and Diameter in Inches is as per Indenting Details.
4.1.9 Record the Receiving details as per Annexure-II “Indenting, Receiving, Codification, Issuance, and Destruction Record of FBD Bag.”
Note: Do a Solid flow monitor test before the use of a fluidized bed dryer.
4.3.0 Storage of New FBD Bag
4.3.1 Place the FBD bag in a Virgin Polybag with a computer-generated label stating “NEW FBD BAG.”
4.3.2 Clean the new FBD bag after receiving it as per the procedure given in step no. 5.1.
4.3.3 Transfer the FBD bag to the storage cabinet meant to store the FBD bag in the Tool Room with the “Cleaned label.”
5.0 Cleaning, Coding, and Issuance of FBD Bag
5.1.0 Cleaning of New FBD Bag
5.1.1 Ensures that Granulation Area is Cleaned as per Product changeover.
5.1.2 Transfer the Bag to the Granulation area in the Virgin Pol bag.
5.1.3 Fit the FBD Bag Hanger to the Pneumatic Cylinder.
5.1.4 Fit the FBD Bag with the Hooks of the Hanger.
5.1.5 Wash the Bag Inside and Outside the Fingers with 150 Liters of Soft Water using the Clean in Place system with NLT 2 Kg/cm2 pressure.
5.1.6 Clean the Bag Inside and Outside the Fingers with 40 liters of Purified Water using the Clean in Place system with NLT 2 Kg/cm2 pressure.
5.1.7 Keep the FBD Bag attached to Hanger for 10 – 15 minutes.
5.1.8 Remove the Bag from the Hanger.
5.1.9 Fit the Bag to FBD and dry it as per SOP for “Operation and Cleaning of Fluidized-Bed Dryer” for 60 minutes at an inlet temperature of 65 ±10°C.
5.1.10 Record the activity in operation, Cleaning, and maintenance records of equipment used for FBD.
5.1.11 Check the integrity of the FBD bag before use.
5.2.0 Issuance of FBD Bag
5.2.1 Collect a new FBD bag from the FBD Bag storage cabinet.
5.2.2 Do Coding of new FBD Bag as per step 5.3
5.2.3 Record the details as per Annexure-II “Indenting, Receiving, Codification, Issuance and Destruction Record of FBD Bag.”
5.3.0 Identification and Coding of New FBD Bag
5.3.1 The identification of the FBD bag will be made as per the product name by stitching the identification tag embroidered with the product name.
5.3.2 Do Stitching of identification tag on the outer edge of the finger bag, which will contact FBT (Finger Bag Tube).
5.3.3 If more than one FBD bag is dedicated to the same product, the coding of the finger bag will be done as in step no. 5.3.4.
5.3.4 If two finger bags of product XYZ are issued simultaneously, the first finger bag will be coded as XYZ, and the second finger bag will be coded as XYZ1.
5.3.5 In case of finger bag destruction, a new finger bag will also be coded as step no. 5.3.1 followed by step no.5.3.2
5.3.6 If the first finger bag of XYZ Product is to be destroyed, then coding of the new finger bag of the same product will be done as XYZ 1 and so on.
5.4.0 Issuance of FBD Bag during Routine Use
5.4.1 Collect the required FBD Bag from Storage Cabinet.
5.4.2 Transfer to Granulation Area in Virgin Polybag.
5.4.3 Check the Cleaning Status from the “Cleaned” Status Label. Check the FBD Bag Integrity Visually.
5.4.4 Record the details as Annexure I, “Usage, Cleaning and Integrity Record of FBD bag.”
6.0 USAGE OF FBD BAG
6.1 Fix FBD Bag to Fluidised Bed Dryer as per SOP for “Operation and Cleaning of Fluidized-Bed Dryer.”
6.2 Dry the Wet Mass as per Batch Manufacturing Record.
6.3 After completion of Batch Drying, remove the FBD Bag from Fluidised Bed dryer.
6.4 Check the Integrity of FBD Bag.
6.5 If FBD Bag Integrity is found not OK, inform the Head of Production and Head of Quality Assurance.
6.6 If FBD Bag integrity is found OK, fix the Bag to a Fluidised Bed Dryer or transfer it for Washing in a Polybag with the appropriate Status Label.
6.7 Record the details as Annexure I, “Usage, Cleaning and Integrity Record of FBD bag.”
7.0 CLEANING OF FBD BAG
7.1 After cleaning, the operator shall visually check the location mentioned in the below pictorial for the cleanliness of the hard-to-access and hard-to-clean areas.
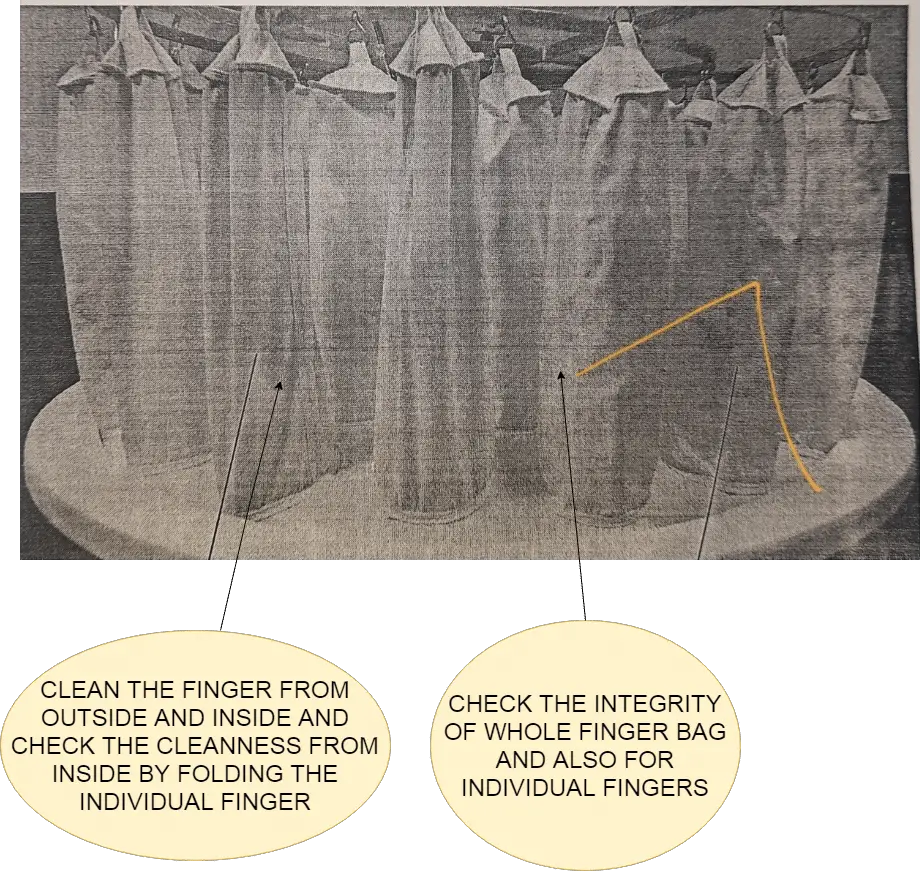
7.2 Batch change over
7.2 1 Shake FBD Bag and collect powder, if any, and transfer it to the Dedicated Container Labeled as “Non-Recoverable.”
7.2.2 Record the details as Annexure I, “Usage, Cleaning and Integrity Record of FBD bag.”
7.3 Procedure for Product Change Over and Serial Cleaning
7.3.1 Shake FBD Bag, collect powder, if any, and transfer it to the Dedicated Container Labeled as “Non-Recoverable.”
7.3.2 Dismantle the FBD bag from Hanger.
7.3.3 Transfer the Bag to Washing Area in Polybag with the appropriate Status Label.
7.3.4 Clean the Bag as mentioned in steps 5.1.3 to 5.1.9
7.3.5 Ensure the Cleaning as per step 7.1 and Transfer the bag in the Virgin polybag to the tool room with the “CLEANED “status label and store it in its respective storage cabinet.
7.3.6 Record the details as Annexure I, “Usage, Cleaning and Integrity Record of FBD bag.”
7.4 Procedure for Re cleaning
7.4.1 Frequency: If Cleaned FBD Bag is not used within one month from Time of Cleaning.
7.4.2 Clean the Bag as mentioned in steps 5.1.3 to 5.1.9
7.4.3 Ensure the Cleaning as per step 7.1 and Record the details as per Annexure I “Usage, Cleaning and Integrity Record of FBD bag.”
8.0 Destruction of FBD Bags:
8.1 Frequency: If the integrity of the FBD Bag is not found satisfactory.
8.2 FBD Bag should be in a clean state before destruction as no product should adhere to it.
8.3 Cut the fingers of the FBD bag with the help of a scissor and record the activity in Annexure-II “Indenting, Receiving, Codification, Issuance and Destruction Record of FBD Bags “
8.4 Dispose of the FBD bag as per the Standard Operating Procedure for the disposal of rejected accessories.
9.0 Abbreviations:
LTD – Limited
PM – Packing material
FBD – Fluidized-bed Dryer
Kg/Cm2 – Kilogram per centimeter square
MOC – Material of Construction
NLT – Not Less Than
SOP – Standard Operating Procedure
FBT – Finger Bag Tube
Annexure-1: For intending and receiving of FBD bag.