1.0 Objective: To lay down a procedure for the management of sieves/ screens / FBD bowl mesh.
2.0 Scope: The procedure is applicable to check the inventory and integrity of sieves/ screens / FBD bowl mesh in the production department.
3.0 Responsibility:
Officer, Executive – Production Department
Manager – Production Department
4.0 Definitions:
NA
5.0 Procedure:
5.1 For Inventory:
5.1.1 On intimation of receipt from stores, collect all the sieve/ screen / FBD finger bags / RMG filter bags from the packing material de-dusting area.
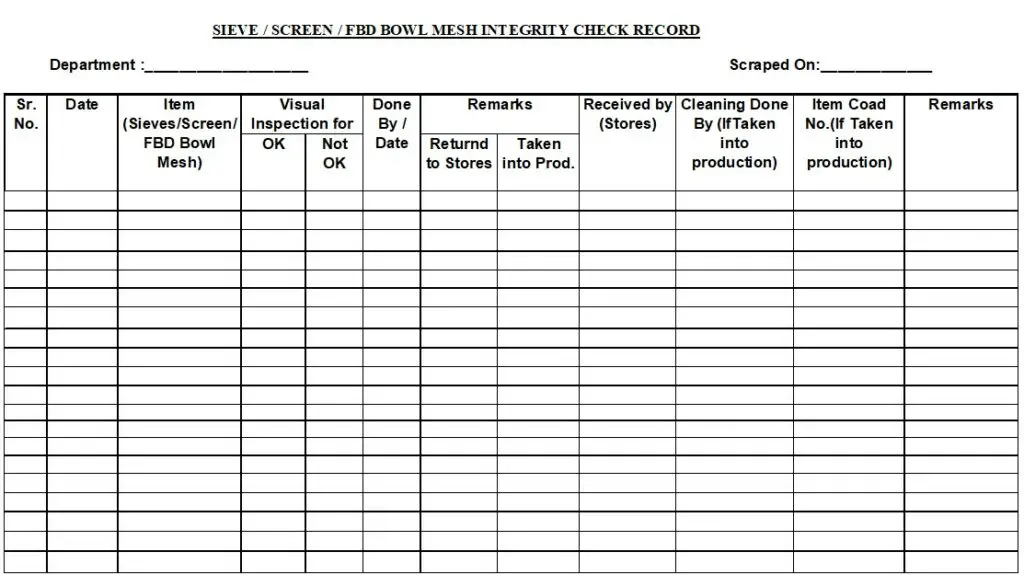
5.1.2 Check the integrity of individual sieve/ screen / FBD finger bags / RMG filter bags against a light source and record the observations in annexure-I.
5.1.3 Remark OK for pass sieve/screen / FBD finger bags / RMG filter bags and Not OK for sieve/screen / FBD finger bags / RMG filter bags which fail the integrity test. Return back the defective sieve/screen / FBD finger bags / RMG filter bags to stores.
5.1.4 Allot code no. as per SOP on “Procedure for Coding Accessories” to the pass sieve/screen / FBD finger bags / RMG filter bags and transfer them to the washroom for cleaning.
Clean the sieve/screen by following SOP No. :
Note: For new sieves/screens received, the sieve/screen shall be cleaned as per SOP “cleaning procedure for all containers, Bins and Sieves” before taking them to the inventory.
5.2 For Integrity:
5.2.1 For sieves:
5.2.1.1 Check the integrity of the sieve and Teflon seal before and after use by holding the sieves against a light source.
5.2.1.2 Scrap the sieve in case of any damage to the sieve.
5.2.2 For screens:
5.2.2.1 Visually check the integrity of the screen before and after use by holding the screen against a light source.
5.2.2.2 Scrap the screen in case of any damage to the screen.
5.2.3 Note:
5.2.3.1 Integrity check of sieve/screen / FBD Bowl Mesh has to be done whenever new sieve/screen are received and recorded in Annexure-I.
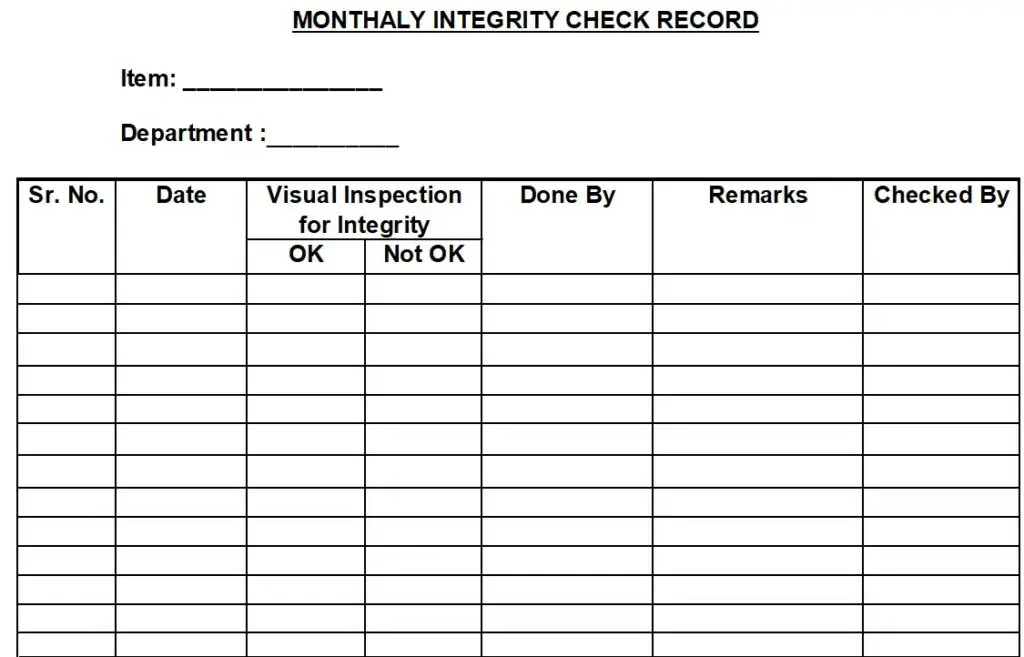
5.2.3.2 Integrity check of sieve/screen / FBD Bowl Mesh has to be done in the first week of every month and record the observations in Annexure-II.
5.2.3.3 If the sieve/screen is found damaged during/after use, Inform to department head and QA.
5.2.3.4 The in-process material shall be kept aside and investigation shall be carried out. Based on the investigation, an action plan is to be prepared.
5.2.3.5 Send the damaged sieve/screen to the engineering department to de-shape before scrapping the sieve/screen.
6.0 Abbreviations:
BMR: Batch Manufacturing Record.
FBD: Fluidized Bed Dryer
SOP: Standard Operating Procedure.
QA: Quality Assurance
7.0 References:
NA
8.0 Annexures:
Annexure –I: Sieve / Screen / FBD Bowl Integrity Check Record.
Annexure –II: Monthly Integrity Check Record.