Standard Operating procedure on Procurement, Usage, and Storage of Primary Standards in Pharmaceutical Quality control Department.
1.0 Objective: 1.1 To lay down a procedure for Procurement, usage, and storage of primary standards
2.0 Scope: 2.1 This procedure is applicable for the Procurement, usage, and storage of primary standards being used in the Quality Control Department in Pharmaceuticals.

3.0 Responsibility: 3.1 Officer/above of the QC department shall be responsible for procurement usage and storage of primary standards.
3.2 Section In charge/ shall be responsible for the execution of this SOP.
3.3 QC Head or his/her Designee shall be responsible for Overall compliance with this SOP.
4.0 Procedure:
4.1Primary Standard:
4.1.1 These are materials which, after drying (or as required) under the specified conditions are recommended for use as a primary standard in standardization of volumetric solution. Or a reference substance as a primary standard that has the appropriate quality within a specified context & is accepted without requiring comparison to another substance.
4.1.2 QC Officer/Executive shall check out the primary standards required for calibration, volumetric solution standardization, and other analysis requirements.
4.1.3 QC Officer/Executive shall provide a list to the section head for procurement of primary standards.
4.1.4 Section head shall be prepared indent for primary standards as per requirement with name.
4.1.4 Mentioned the Grade, manufacturer, and quantity on indent to the procurement of primary standards.
4.1.6 Analytical reagent grade (certified) materials of commerce must be used.
4.1.7 After receiving these chemicals QC Officer/Executive shall ensure that it is labeled properly, indicating the name of the chemicals and purity with their grade.
4.1.8 Check the container seal for its integrity, if any damage or leakage is found, the same shall be sent back. If the container is found OK use the same as a primary standard.
4.1.9 Primary standards shall be Stored at the temperature mentioned on the label of the container.
4.1.10 The date of opening shall be mentioned on the container when the bottle is opened, use before the date must be assigned 3 years for solids, 2 years for liquid, and 6 months for hygroscopic materials.
4.1.11 COA of Respective primary standards shall be downloaded/received from its manufacturers, websites, or vendors.
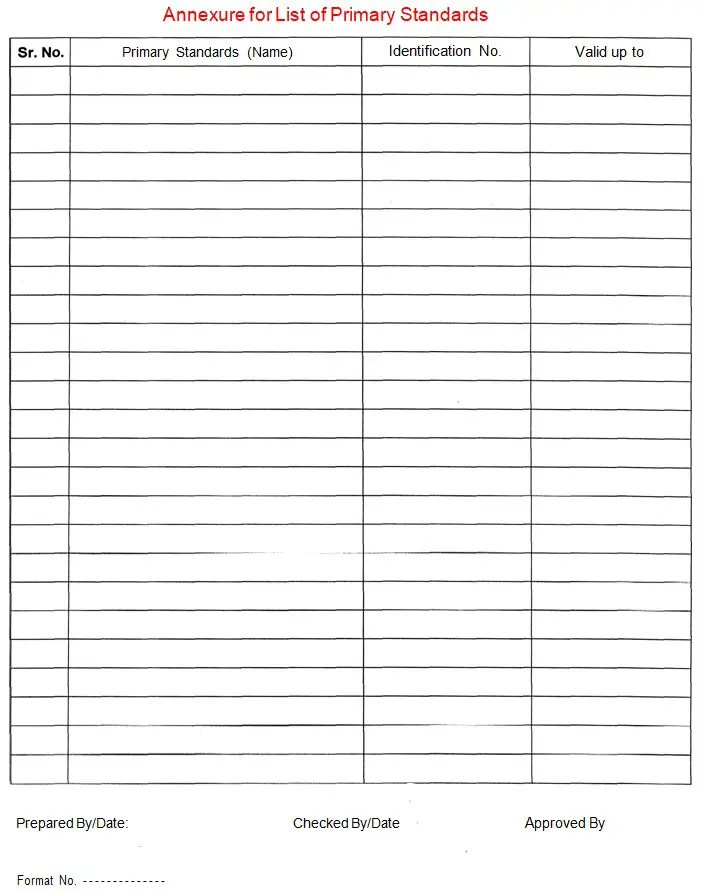
4.1.12 Enter the details of all primary standards with unique ID No. in Annexure-l of this SOP as per the below pattern.
PS-XXX
Where,
PS: denotes to Primary Standard,
XXX: denotes Unique ID No. for particular primary standards.
4.13 Containers of the primary standard shall be labeled with ID no. as per the below pattern.
PS-XXX-ZZ
Where,
PS: denotes to Primary Standard,
XXX: denotes to Unique ID No. for particular primary standards,
ZZ: denotes to Alternate No. for the next container of the same primary standard.
Example: PS-001-01 for the First container of the primary standard and PS-001-02 for the Second container of the same primary standard and so on.
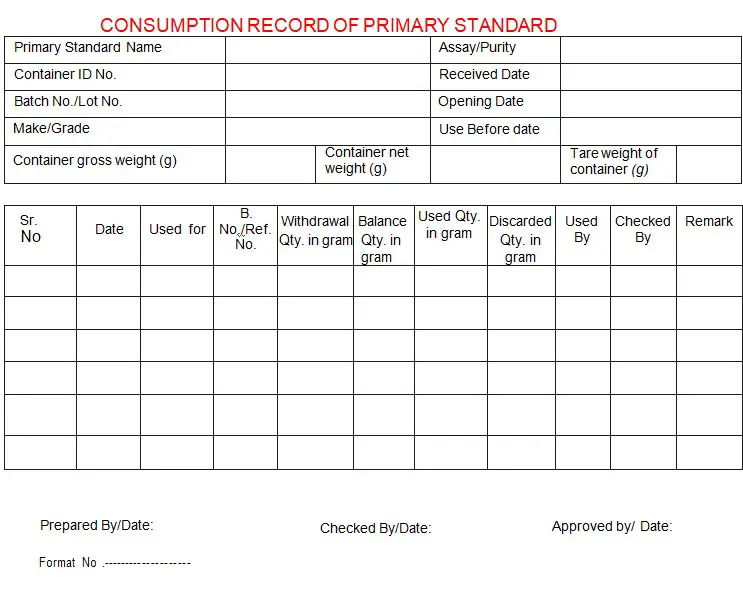
4.1.14 Maintain the primary standards consumption record as per Annexure-ll of this SOP.
4.1.15 Dispose of the expired primary standard as per SOP No. QC/CHE/021 for Disposal of Residual sample/Leftover material
Related SOP: SOP on Receipt and Handling of Laboratory Samples
4.2 Precaution:
4.2.1 Take only the required quantity of the primary standards from the respective containers and do not add the remaining Quantity of primary standards in the original container.
4.2.2 If the respective primary standard is stored at below 25°C, allow it to attain the ambient temperature before use.
4.2.3 Never use any external tool, such as a spatula, glass rods, etc. into the container of the primary standards & if required, clean butter paper use only.
4.2.4 Always use PPES during the handling of primary standards.
5.0 Reference:
As per GMP Guideline/IP/BP
6.0 Annexures/Attachment:
Annexure-l: List of Primary Standards
Annexure-II: Consumption record of primary standards
7.0 Distribution:
Master Copy: Quality Assurance Department.
Controlled Copy: Quality Control Department
Display Copy: Chemical Analysis Lab.
8.0 Abbreviations:
SOP: Standard Operating Procedure
QC: Quality Control
LR: Laboratory Reagent
A.R: Analytical Reagent
G.R: General Reagent
°C: Degree centigrade
PPES: Personnel Protective equipment
ID: Identification
COA: Certificate of Analysis
IP: Indian pharmacopoeia
BP: British Pharmacopoeia