Change control is Managing to control a change in a process/procedure/system through proper and scientific justification, review, approval, implementation, follow-up, and closure.
EU GMP Annex 15 Guidelines explain “change control” as:
“The given statement describes a formal process in which qualified individuals from relevant fields review proposed or actual changes that could potentially affect the validated status of facilities, systems, equipment, and processes. The purpose of this process is to determine the necessary actions that need to be taken in order to confirm and document in such a way that the system is being maintained in a validated state.”
EU GMP Chapter 5.23 Guidelines says regarding the handling of changes:
“Significant modifications to the manufacturing method, including any change in
equipment or materials, which may affect the quality of the products and/or the reproducibility of the method should be validated.”
CFR also release two brief notes on change control under “CFR 211.100 Written procedures; deviations” and “211.160 General requirements“.
Objective of Change Control System?
The objective of change control is to provide a mechanism for ongoing process optimization/system improvement and ensure continuous process control throughout the stage.
What types of changes can be covered?
Suggested changes to:
- Processing Steps
- Specifications & Test Procedures
- Facilities and equipment
- Support Systems (Includes computer hardware and software)
- Key/starting materials (includes vendor)
- Packaging materials/configuration
Purpose of the Change Control Program in Pharmaceuticals?
- To prevent unauthorized changes to a validated system.
- To evaluate proposed changes against development and technology transfer documents.
- To identify and evaluate all proposed changes to assess their potential effects on the manufacturing process.
- To determine if, and to what extent, revalidation is needed
- Ensure that all documents affected by changes are promptly revised.
- To determine the impact of changes on the critical chemical, physical and microbiological attributes of the product, such as assay, impurity profile, stability, and physical characteristics.
The probable effect of changes to improve process yields?
Such proposed changes should be evaluated carefully to determine if they result in new or higher levels of impurities/degradation products
The impurity/degradation profile of resulting batches should be comparable.
Could process changes affect analytical methods?
Yes! Changes to the process should also be considered to ensure that they do not impact the analytical procedure. When impurities or degradation products are present at higher levels, they can cause more interference and have a greater effect. Therefore, it is important to use analytical methods that have been updated as necessary to accurately detect and measure these impurities and degradation products.
Follow-up is required after changes to the manufacturing process are implemented.
In case, the manufacturing process changes affect the critical attributes of the API,
- Notify dosage form manufacturers
- Notify Regulatory bodies, if the mfg process is filed
- Revalidation (where required)
- Prompt revision of other documents affected.
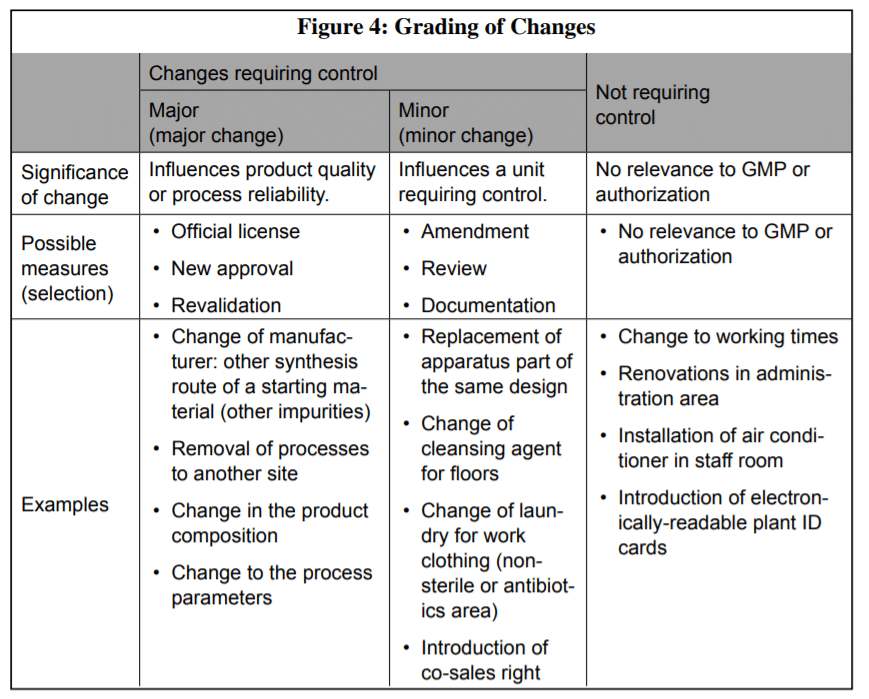
Classify or categorize Change control in Pharma?
a) The change control system should provide for a classification system to evaluate changes in :
- Raw Materials, Intermediates, Packing Materials
- Manufacturing Sites
- Scale of Manufacturing
- Manufacturing Equipment
- Production process
- Specifications-STPs
- Editorial changes
b) The classification procedure should be used to determine what level of:
- Testing,
- Validation
- Documentation is needed to justify changes to a validated process
How do we, therefore, categorize changes?
We can categorize it into Minor, Moderate, or Major, depending on the nature and extent of the changes, and the effects that these changes could impart on the process/product.
Read Also: Validation Process

Panks Pamyal is a Author and Editor at Pharmaguddu.com. He Worked in Top Pharmaceuticals MNCs in India had a more then 10 years experience in Quality control department. He Delivering most valuable insights and knowledge through this website.
