Definition: “Extraction involves using a liquid solvent to remove active constituents from a solid or liquid substance. Or The separation of medicinally active portions of plant or animal tissues from the inactive or inert components by using selective solvents.”
The extraction process has the following stages:
- The solvent penetrates into the solid matrix.
- The solute dissolves in the solvents.
- The solute is diffused out of the solid matrix.
- The extracted solute is collected.
Some important terms
Menstruum– The solvent used for extraction is known as a menstruum.
Marc-Marc” refers to the inert insoluble material left behind after extraction.
Galenical-“Galenical” describes a medicine that is prepared by extracting one or more active constituents from a plant.
Steps involved in the extraction of medicinal plants
- Size reduction
- Extraction
- Filtration
- Concentration
- Drying
Solvent/menstruum used in extraction processes
1. Water
- It serves as a polar solvent and finds usage in extracting a broad spectrum of polar compounds.
- Solvent for protein, coloring matter, gums, glycoside, sugar, alkaloids salts, enzymes, and many organic acids and salt.
- Wax, fat, fat oil, and most alkyl halides are insoluble in water.
Advantage
- It is cheap, non-toxic, non-flammable, and highly polar.
Disadvantage
- While solvent action dissolved a wide range of substances that are undesirable.
- The growth of molds and bacteria is easy therefore needs preservatives.
- Hydrolysis of the drug.
- Degradation of the product because of fermentation.
2. Alcohol
- It is also polar and can mix with water. It can extract polar secondary metabolites.
- It serves as a solvent for alkaloids, alkaloid salts, glycosides, volatile oils, and resins.
- Does not dissolve albuminous matter, gum, wax, fat, fixed oil, and sugar.
Advantage
- No mold and bacterial growth if the concentrate is 20% or above 20% (self-preservative)
- Dissolve selective active constraint of drug
Disadvantage
- It is costly and flammable and volatile
3. Chloroform
- Nonpolar solvent
- Useful in the extraction of compounds such as terpenoids, flavinoids, fats, and oils.
Advantage
- Colorless, sweet smell, and soluble in alcohol.
- Also absorbed and metabolized in the body.
Disadvantage
- Sedative and carcinogenic properties.
4. Ether
- Nonpolar solvent
- Useful interaction of compounds such as alkaloids, terpenoids, coumarins, and fatty acids
Advantage
- Miscible with water
- Low boiling point
- Tasteless
Disadvantage
- Highly volatile and flammable in nature
5. Ionic liquid (green solvent)
- It is highly polar and extremely heat stable.
- It is miscible with water and other solvents.
Advantage
- The excellent solvent that attracts and transmits microwave.
- it is non-flammable.
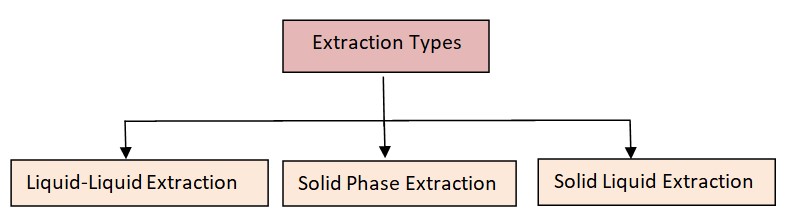
Types of Extraction ( Classification)
Extraction involves using a liquid solvent to remove active constituents from a solid or liquid substance. it’s done using menstruum.
1. Liquid-Liquid Extraction (Solvent extraction)
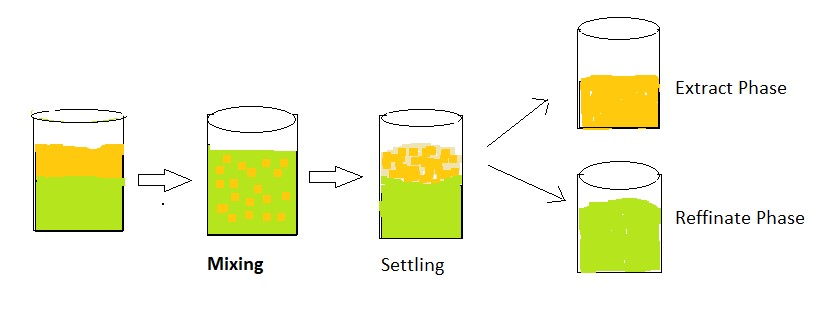
- In this extraction of the components of a liquid mixture are separated by contacting them with a suitable insoluble solvent which preferentially dissolves one or more components.
- The separation of the components from the liquid mixture depends on how the components distribute themselves between two liquids that don’t mix.
- In this extraction, the feed solution is phase 1 and the solvent used for extraction is phase 2.
- Both the feed and solvent form a homogenous mixture which is separated by contacting them with one another to separate out one of the two liquids preferentially.
- This type of extraction is most widely used to separate actives and aromatic compounds from plants.
2. Solid Phase Extraction (SPE)
- Solid phase extraction is used for the isolation, enrichment, and purification of components from aqueous solutions depending upon their physical and chemical properties.
- It involves contacting aqueous samples with a solid phase or sorbent, where the component under consideration is adsorbed on the surface of the solid phase prior to elution.
- The extract amount is negligible compared to the quantity of analyte in the sample.
- Analytical laboratories use this type of extraction process.
3. Solid-Liquid Extraction(SLE)
- Solid-Liquid Extraction also called leaching
- Solid-liquid extraction is the process of taking out specific parts from a mix of solids by allowing the solid substance to touch a liquid solvent. The solvent dissolves these specific parts.
- Leaching is used to create a concentrated solution of an active component or to separate an insoluble part from a soluble material it’s mixed with.
- Examples- maceration, percolation, and supercritical fluid extraction.
Methods of Extractions
- Maceration
- Percolation
- Decoction
- Digestion
- Infusion
- Soxhlet extraction
- Microwave-assisted extraction
- Sonication extraction
- Accelerated solvent extraction
- Supercritical fluid extraction
1. Maceration
- Maceration is a process where plant materials are soaked in a sealed container with a specific solvent, usually in powder or coarse form.
- The setup is left at room temperature (+25°C) for at least 72 hours with shaking.
- This softens and breaks the plant’s cell wall to release soluble bioactive substances. After 3 days, the entire mixture is filtered by pressing and sieving using Whatman no. 1 filter paper.
- The choice of solvent is important in determining the type of phytochemicals extracted from the samples. This method is suitable for heat-sensitive plant materials.
There are two types of maceration:
a. Simple maceration(for organized drugs)
- The crudely organized drug is placed in a closed vessel and filled with the entire volume of solvent.
- It is shaken occasionally for 7 days, then the liquid is strained, pressed, and mixed.
- The final preparation is filtered to remove any insoluble content, and the volume is not adjusted. Examples include the tincture of orange and the tincture of lemon.
b. Modified maceration(for unorganized drugs)
- The drug is placed in a vessel and 4/5 of the solvent is added.
- It is shaken occasionally for 2 to 7 days, and then the liquid is decanted.
- The marc is not pressed.
- The remaining 1/5 of the solvent is added to adjust the final volume. Examples include the tincture of tolu and the compound tincture of benzoin.
2. Percolation
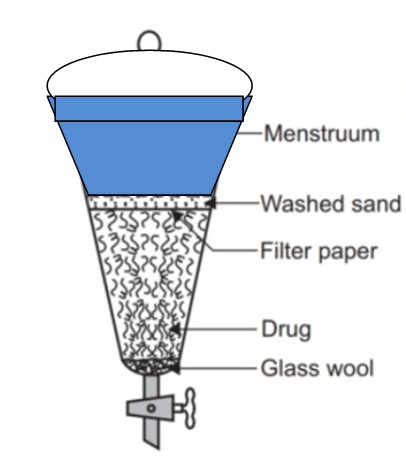
- Percolation is another commonly used method for extracting phytochemicals.
- A percolator, which is a narrow cone-shaped vessel open at both ends, is used.
- The solid ingredients are moistened with a specified amount of solvent and left to stand for about 4 hours in a closed container.
- The wet mass is then packed, and the additional solvent is added to form a shallow layer above the mass.
- After 24 hours of maceration, the liquid is allowed to slowly drip out through the outlet of the percolator.
- The resulting percolate is mixed to reach about three-quarters of the required volume.
- The macerated product is pressed, and the expressed liquid is added to the percolate.
- Properly prepared solvent is added to achieve the desired volume, and any excess liquid is removed by filtration or decanting.
3. Decoction
- Decoction involves boiling the crude product in a specified volume of water for a defined period, then cooling and straining or filtering it.
- This method is suitable for extracting water-soluble, heat-stable metabolites.
- The starting ratio of crude product to water is fixed, and the extract is concentrated to one-fourth of its original volume by boiling.
- A decoction is particularly useful for extracting compounds from hard plant parts like roots and barks and yields more oil-soluble phytochemicals compared to maceration and infusion.
4. Digestion
- Digestion is a maceration method that includes the application of gentle heat during extraction.
- It is used when a moderately raised temperature is needed to increase the efficiency of the
- menstruum.
- In this process, the drug is extracted by heating at a particular pressure to increase the penetration
- power of the menstruum, so that there is complete extraction of the drug.
- The apparatus used is known as ‘Digestor’ which is a vessel made up of metal.
- The whole of the drug is placed in the covered digestor and bolted with the help of nuts.
- The drug is heated under specified conditions of temperature and pressure in a vessel known as a ‘Digestor.’
5. Infusion
- Infusion is prepared by macerating the crude drug for a short period with either cold or boiling water.
- Processes-Drug is placed at the bottom of the pot, then add water/hot water and stirred three to four times, stand it for 15 min, filtering off the liquid without pressing the marc.
- Fresh infusion should be used within 12 hours after preparation to avoid spoilage due to fungal and bacterial growth.
- Concentrated infusion- It is prepared by double/triple maceration processes and is 8 times stronger than fresh infusion. Alcohol (20-25% concentration) used as solvent therefore can be stored for a longer period of time. Eg- infusion of Quassia, conc. Infusion of chirata.
6. Soxhlet Extraction (Hot Continuous Extraction)
- Soxhlet Extraction, also known as Hot Continuous Extraction, is an automatic method that continuously extracts the herb with fresh solvent using the principle of reflux and syphoning.
- Principle-soxhlet utilizes the principle of reflux and syphoning to continuously extract the herb with fresh solvent.
- The soxhlet extraction method is suitable when-Menstruum has less penetration into the cellular tissue and active constituents are not readily extracted with a solvent.
- For example- fixed oil from seeds, Alkaloids from drugs are extracted by a soxhlet extraction process using benzene, chloroform, petroleum, ether, etc.
- Note- the high temperature and long extraction time in the soxhlet extraction will increase the possibilities of thermal degradation.
Related: Mixing – Pharmaceutical Engineering
Apparatus
The Extraction apparatus consists of:
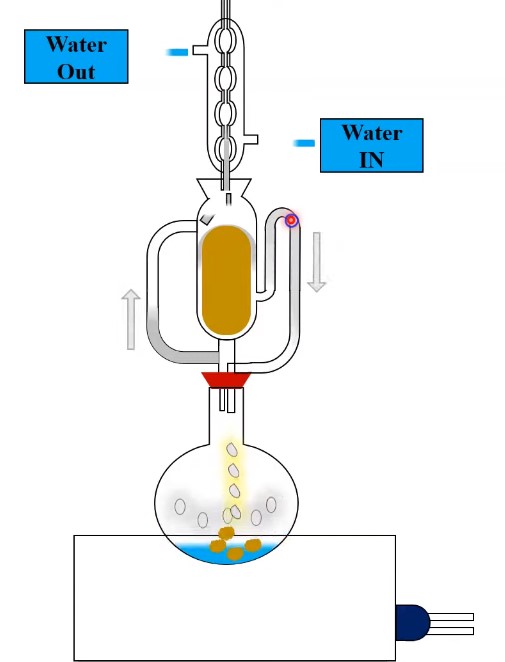
- Flask (round bottom) containing the boiling solvent.
- The Soxhlet extractor is a device used for extracting drugs. In this extractor, we pack the drug to be extracted. There is a side tube in the extractor that carries the solvent vapors from the flask to the condenser. Additionally, there is a siphon tube that transfers the extract from the Soxhlet extractor back to the flask.
- A condenser in which the vapors of the solvent are condensed again into a solvent.
Procedure
- Placed finely ground crude drug which is packed into a porous bag/thimble made of filter paper in Soxhlet extractor.
- The solvent is placed in the flask and boiled.
- When solvent is boiled on heating the flask it gets converted into vapors. These vapors enter into the condenser through the side tube and get condensed into hot liquid which falls on the
column of the drug. When the extractor gets filled with the solvent the level of the siphon tube also raises up to its top. The solvent contains active constituents of the drug in the siphon tube. - Siphon over and runs into the flask thus emptying the body of the extractor.
- The extractor continuously fills and empties its body. The flask holds the active part of the drug, while the solvent keeps evaporating repeatedly. They repeat the filling and emptying process in the extractor until all the drug is used up.
- Usually, they repeat this process around 15 times to completely use up the drug.
Applications of Extraction
- The extraction process is used in the separation of antibiotics and protein recovery.
- It is used to recover high-boiling components such as phosphoric acid, boric acid, and sodium hydroxide from aqueous solutions.
- It is used to obtain therapeutically active constituents from plant parts and to eliminate inert material.
- It is used to isolate renin enzymes and insulin hormones from animal sources.
- Gelatin is produced by the conversion of skin and bone collagen by treatment with lime or diluted acid and is further extracted with warm water.
- It is used in the extraction of fixed oils from seeds.
A brief Explanation of various extraction methods
| Extraction Method | Description | Applications | Temperature (°C) | Pressure (atm) |
| Maceration | Soaking the plant material in a solvent (usually water, alcohol, or a mixture) for an extended period. | Herbal preparations, tinctures, infusions | Room temperature | Atmospheric |
| Percolation | Passing a solvent through a packed bed of the plant material to extract soluble constituents. | Preparation of herbal extracts, tinctures | Room temperature | Atmospheric |
| Soxhlet Extraction | Continuous extraction using a cycle of boiling, condensation, and solvent reflux through a thimble. | Extracting lipids, essential oils, and alkaloids | Boiling (80-100) | Atmospheric |
| Steam Distillation | Distilling plant material using steam to extract essential oils and other volatile compounds. | Production of essential oils | Boiling (100) | Atmospheric |
| Supercritical Fluid Extraction (SFE) | Using supercritical fluids as a solvent to extract compounds without damaging heat-sensitive substances. | Extracting plant compounds, pharmaceuticals | Above critical temperature (31-100) | Above critical pressure (74-400) |
| Solvent Extraction | Shaking or stirring the plant material with a solvent, and separating the extract from the residue. | Isolating active compounds from natural sources | Room temperature | Atmospheric |
| Carbon Dioxide (CO2) Extraction | Using pressurized carbon dioxide to extract various compounds, leaving no solvent residues. | Extracting essential oils, herbal extracts | Above critical temperature (31) | Above critical pressure (74) |
| Cold Pressing | Mechanical pressing of fruits or seeds to obtain oils without external heat application. | Production of cold-pressed oils | Room temperature | Atmospheric |
| Ultrasonic Extraction | The increased extraction efficiency of various compounds | Increased extraction efficiency of various compounds | Room temperature | Atmospheric |
| Microwave-Assisted Extraction (MAE) | Using microwave energy to heat the solvent and facilitate the extraction of compounds. | Efficient extraction of bioactive compounds | Varies (depending on solvent) | Atmospheric |

Panks Pamyal is a Author and Editor at Pharmaguddu.com. He Worked in Top Pharmaceuticals MNCs in India had a more then 10 years experience in Quality control department. He Delivering most valuable insights and knowledge through this website.
