SOP for Cleaning and Operation of Outside Micrometer
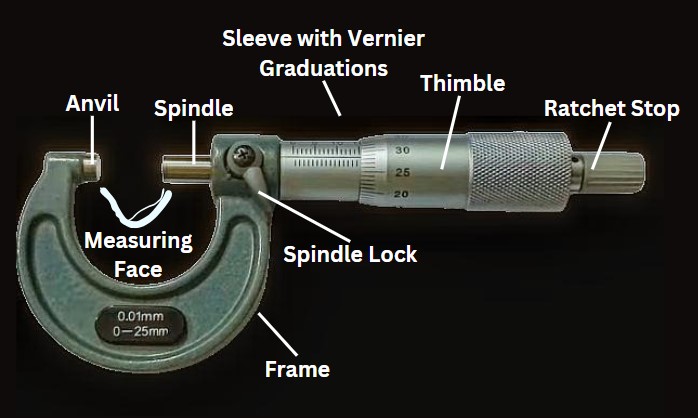
Standard Operation Procedure for Cleaning and Operation of Outside Micrometer in Pharmaceuticals. Purpose: To lay down a procedure for cleaning & operation of Outside Micrometer. Scope: This procedure is applicable for all Outside Micrometers used at our organization. Responsibility: Accountability: Quality Manager Procedure: Definition: Outside Micrometer (External Micrometer): It accurately measures the outside diameter, length, … Read more