Learn About Pharmaceutical Suspension- Classification, Composition, Theory, Formulation/Preparation, Evaluation, Types, Advantages, and Disadvantages.

Definition: Pharmaceutical Suspension is a biphasic system that consists of solids finely dispersed into a liquid medium. It is a coarse dispersion in which the active ingredient as the internal phase is dispersed uniformly throughout the external phase with the help of a suspending agent. The particle size of the solute varies from 0.5 to 5 pm. A proper choice of excipients such as surfactants, viscosity modifiers, wetting agents, etc. is important. Particle size and its distribution in a suspension dosage form are critical and significantly affect the bioavailability and pharmacokinetics of the formulation. Suspension is usually formulated for drugs that are water-insoluble or poorly soluble such as Prednisolone suspension. They are also formulated to prevent the degradation of the drug or to improve its stability as in the case of Oxy-tetracycline suspension. Suspensions also mask the bitter or unpleasant taste of drugs such as Chloramphenicol palmitate.
Classification of Pharmaceutical Suspension Based on the route of administration:
Pharmaceutical Suspension formulations are classified as:
Topical suspensions: They are applied on the skin externally for local effect. Examples include Calamine lotion.
Oral suspensions: Suspensions are administered orally for systemic effect. Examples include Paracetamol suspension and Ibugesic suspension.
Parenteral administration: Suspensions are administered intravenously or subcutaneously for prompt effect. Examples include Penicillin procaine suspension, Methyl Prednisolone injection.
Intramuscular Suspensions: Suspensions arc formulated as vaccines for immunization Example includes Hepatitis a vaccine for intramuscular administration.
Pharmaceutical Suspension classifications based on a percentage of solute:
Based on a percentage of solute, two types of suspension in Pharmacy as below:
Dilute suspension: Suspension consists of 2 to 10% w/v of solid particles. The example includes Cortisone acetate suspension.
Concentrated suspension: Suspension consists of 50% w/v solid particles. An example includes Zinc oxide suspension.
Suspensions are also classified depending on the electrokinetic property of solid particles:
Flocculated suspension: The particles in flocculated suspensions form loose aggregates with a network-like structure. Such suspensions show a high rate of sedimentation but the sediments are loosely packed and a hard cake is not formed. There is no difficulty to re-disperse such flocs. Such suspensions are not pleasing in appearance and tend to adhere to the walls of the container. An example includes antibiotic suspensions.
Deflocculated suspension: Suspensions consist of uniformly scattered particles in the aqueous phase. In deflocculated suspensions particles exist as separate entities and exhibit a slow rate of sedimentation. Sediments tend to form a hard cake which is difficult to re-disperse. Such suspensions are pleasing in appearance and don’t stick to the sides of the container. An example includes Sodium silicate soil suspension.
Based on the size of solid particles, Pharmaceutical suspensions are classified as:
Colloidal suspensions: Suspensions have a particle size of less than I micron meter.
Coarse suspensions: Suspensions with particle size greater than 1 micron in diameter. Nanosuspensions: Suspensions with fine narrow dispersion of particles to a size range of 100-300 nm. An example includes Fenofibrate suspension (Tri Cor’).
Related: Liquid Syrup Types and Manufacturing Process
Composition of Suspensions:
Pharmaceutical Suspension formulations consist of the following excipients which help in making a stable and robust formulation.
Wetting agents: They are added to decrease the contact angle between the dispersed solid and the dispersion medium thus aiding in the spreading of solute into the liquid medium by replacing the adsorbed air around the solid particle. Hydrophilic solutes are easily wetted by water but hydrophobic drugs are easily wetted by non-polar liquids. Wetting agents are added in a concentration of less than 0.5%. They tend to reduce the surface tension between the solid particle in the suspension thus increasing their solubility: The most common examples include Elycslin, propylene glycol, and alcohol. Methylcellulose, acacia, pectin, sodium lauryl sulfate, polysorbate 80, poloxamers, and Pluronics.
They are also used in liquid formulations. They work well with the aqueous phase. With non-aqueous liquids, mineral oil is commonly used. Wetting agents consist of branched hydrophobic chains along with central hydrophilic groups or short hydrophobic chains with hydrophilic end groups. The hydrophile lipophile balance (HLB) of wetting agents is generally 7-9.
Flocculating Agents: Flocculating agents are added to impart the right zeta potential to particles so that flocculation occurs in the formulation. Flocculating agents can be water-soluble organic polymers or inorganic salts. They tend to neutralize the surface charge of suspended particles, shrink the ionic double layer, or bridge the suspended particles. Examples include Aluminum chloride and electrolytes.
Surfactants: Surfactants or surface active agents are added to decrease the surface tension between the drug and the aqueous medium. They also serve several other purposes such as preventing crystal formation in suspension, wetting the dry solid powders, improve syringability. They have a lipophilic tail and a hydrophilic head. Usually, non-ionic surfactants are used but ionic surfactants can also be used depending on the solute. Some of the examples include sorbitan monooleate (Span 80) in a concentration of 0.05% to 0.25% and polyoxyethylene sorbitan monooleate (Polysorbate 80) in a concentration of 0.1 % to 0.5% are commonly used surfactants. Polysorbate 80 is a non-ionic surfactant that does not change the pH of the medium. It is safe for oral administration.
Flavoring and coloring Agents: Suspensions are required to have high patient acceptability, especially for the pediatric age group. Flavorants are added to enhance palatability by stimulating the sensations of flavor. Natural flavors include camphor oil, eucalyptus oil, sandalwood oil, essential oils, and oleoresins while synthetic flavorings are methyl salicylate, vanillin, lactones, etc.
Coloring agents are added to impart specific colors for easy identification of the formulation or to give an aesthetic look to the formulation. Colorants are obtained from natural or synthetic sources. Plant colors include chlorophyll, anthocyanins, carotenoids, and betalains. Synthetic coloring agents include brilliant blue (blue), indigo carmine (blue), amaranth (red), tartarazine (yellow), and titanium dioxide (white). They are used within the range of (0.0005 % to 0.001%).
Suspending Agents: Certain hydrophobic drug molecules, even after the removal of adsorbed air, are not wetted easily. Therefore surface active agents are required to reduce the surface tension between the particles and the vehicle. Suspending agents increase the viscosity of the continuous medium and delay the rate of settling of suspensions; examples include xanthan gum, carrageenan, sodium carbon, methylcellulose, and carbopol. Suspending agents also form a film around particles and decrease inter-particle attraction, the example includes bentonite.
Buffers and pH adjusting agents: Buffers are added in suspension formulations to resist change in pH, therefore imparting stability to the composition. Generally, the pH of a suspension formulation lies in the range of 7.4 to 8.4. The most commonly used buffers include salts of weak acids such as carbonates, citrates, gluconates, phosphate, and tartrates.
Preservatives: Preservatives are chemical substances that are added to pharmaceutical compositions to prevent decomposition by microbial growth or any undesirable chemical change. They extend the shelf life of a drug. Some of the natural preservatives include salt, lemon juice rosemary extract, etc. The chemical preservatives include sodium benzoate, benzoic acid, sodium nitrite potassium sorbate, sulfur dioxide, and sodium sorbate.
Solvents Suspensions are biphasic formulations; therefore for proper wetting of solutes, solvents such as alcohol, glycerin, polyethylene glycol, and polypropylene glycol are used. They reduce liquid air interfacial tension thus facilitating wetting.
Osmotic agents: They are added to produce osmotic pressure comparable to biological fluids. They are particularly added in ophthalmic or injectable suspensions. The most commonly used osmotic agents are dextrose, mannitol, sorbitol, sodium chloride, sodium sulfate, and glycerol.
Sweeteners: Sweeteners are added to mask the bitter taste of the drug. They are used for taste masking of bitter drug particles. Bulk sweetening agents employed in oral suspensions are sucrose, liquid glucose, glycerol, and sorbitol while artificial sweetening agents include saccharin sodium, ammonium glycyrrhizinate, and aspartame. A bulk sweetener is used at a concentration of 15-70 w/w of the total weight of the suspension. The amount of artificial sweetening agents should be between 0 to 5 g/100 mL of suspension.
Humectants: Humectants are substances that are added to the suspensions to reduce the loss of moisture. Some examples include propylene glycol, glycerol, polyethylene glycol, urea, and silicones. Natural humectants include glycerine and honey. The total quantity of humectants used in suspensions lies in the range of 0-10 % w/w.
Antioxidants: Antioxidants are substances that inhibit oxidation. Natural antioxidants include vitamins, carotenoids, and phenolic compounds from fruits and vegetables. Synthetic antioxidants include butylated hydroxyl toluene, propyl gallate, butylated hydroxyanisole, cysteine, and sodium metabisulfite.
Theory of Suspensions used in Pharmacy:
Surface area theory:
Surface area theory In suspension formulations, a large surface area is observed due to a finely divided solid, which in turn is associated with a large amount of free energy on the surface. The expression of surface free energy and the surface area is given as:
yG=g y A
Where yG is the increase in surface free energy, y A is the increase in surface area, and g is the interfacial tension between the solid particles and the dispersion medium.
Surface potential:
In suspensions, the surface potential exists when disperse solid particles carry charge in relation to their surrounding dispersion medium.
Electric double layer:
The electric double layer is formed in order to neutralize the charged particles in a suspension. Due to this electrokinetic property, the biphasic system shows the formation of:
(i) a tight layer due to ions from dispersed particles and counter ions from the dispersion medium
(ii) a diffusion layer
(iii) electro-neutral layer. The difference in electric potential between the actual surface of the particle and the electro-neutral region is referred to as Nernst potential which is controlled by the electrical potential at the surface of the particle due to the potential determining ions and has little effect on the stability of emulsions. The potential difference between the ions in the tightly bound layer and the electro-neutral region is referred to as zeta potential and has a significant effect on the stability of the emulsion. Zeta potential governs the degree of repulsion between adjacent, similarly charged dispersed particles. If the zeta potential is reduced below a critical value, the force of attraction between particles succeeds leading o coalescence. The figure below explains the different potentials that a particle bears.
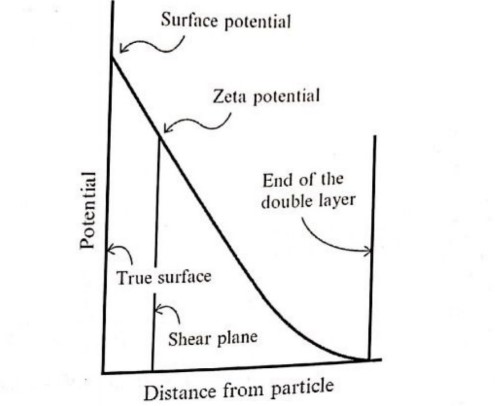
Formulation/ Preparation of Pharmacy Suspensions
On a small scale, suspensions are prepared by grinding or levigating the insoluble solute with water in the mortar to form a smooth paste. The aqueous phase may contain a wetting agent, suspending agent, a flocculating agent, a thickening agent, etc. Once the slurry is ready, the rest of the aqueous phase is added to make the volume to the desired quantity. At large scale, basically, two methods are used:
Dispersion method: In the dispersion method, the solute is uniformly dispersed into the vehicle. Dispersion can be carried out with irradiation with ultrasonic waves. The method produces line particles without the use of foreign substances in the system. It is favorable when the solute is present as a precipitate. Electrical Dispersion is also carried out when two wires are immersed in a liquid vehicle containing an electrolyte or steric stabilizer. The vapors of the solute condense and are dispersed in the liquid medium as small particles. This method is particularly useful for suspensions containing metal ions.
Dispersion is also carried out using chemicals used to peptize the dispersed particles. For example, positively charged particles are peptized by acids. Vanadium pentoxide and hydrogen are also used to cause peptization.
Condensation method: In the condensation method, a macromolecular solution of solute is poured into a liquid in which the solute is practically insoluble. As the solubility of solute is low, it is precipitated as small particles. The system is then stabilized with electrolytes or alkalis. An increase in temperature can either promote or retard the formation of such small particles.
Generally, three approaches are used to formulate a Pharmaceutical suspension:
- Use of structured vehicle
- Use of controlled flocculation
- Flocculation in structured vehicles
Structured vehicles: Structured vehicles are the aqueous solutions of natural and synthetic gums. They are added to increase the viscosity of the dispersion medium. They are also called thickening or suspending agents. Structured vehicles are used for deflocculated suspensions. These structured vehicles entrap the particles and reduce their sedimentation. Some examples include methyl cellulose, sodium carboxy methyl cellulose, acacia, gelatin, and tragacanth. Suspensions prepared with structured vehicles show retarded dissolution as particles are adsorbed onto the surface. Such suspensions are difficult to pour. They are not formulated for parenteral formulations due to a lack of syringability.
Controlled flocculation: Controlled flocculation is carried out by adding flocculating agents, which might belong to the category of electrolytes, surfactants, and polymers. According to the Schulze-Hardy rule and Derjaguin-Landau-Verwey-Overbeek (DLVO) theory, suspensions are flocculated by the addition of electrolytes carrying a charge opposite to the charge of the particles.
High molecular weight polymers are also added to form a bridge between particles so that flocculation occurs in a controlled manner. Examples of polymers include polystyrene and polyvinyl alcohol. Adsorption of surfactants on the surface of the dispersed particle also brings about flocculation, example includes sodium dodecyl sulphate, an ionic surfactant on the alumina surface.
Flocculation in structured vehicles
Sometimes suspending agents are added to the flocculated suspension to retard sedimentation. examples include carboxymethylcellulose (CMC), carbopol 934, veegum, and bentonite.
The settling of particles in a suspension is expressed through Stoke’s law:
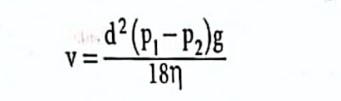
v= Sedimentation rate, d= Particle size, g = acceleration due to gravity, p1 = particle density, p2 = vehicle density, and n= vehicle viscosity.
Evaluation of Pharmacy Suspensions
A. Physical stability: Physical stability of a suspension covers organoleptic properties such as appearance, color, odor, and taste. However, specific gravity, sedimentation rate, sedimentation volume, and microscopic examination for crystal size and aggregate formation are also assessed.
B. Chemical stability: The chemical stability of a suspension involves the study of the determination of uniform drug distribution, degradation of the active ingredient, viscosity change, pH, and adsorption of preservatives onto drug particles. In general, the important evaluation parameters for a suspension formulation include:
1. Visual inspection: For visual inspection of suspensions, a sign of physical instability is observed which is indicated by the formation of floccules that do not disperse readily on gentle shaking. A change in color may indicate chemical degradation or microbial contamination of the formulation. With a visual inspection, the ingredients and the final products are carefully examined for purity and appearance. This physical evaluation is required (or patient adherence and compliance, therefore, the formulation should be elegant in appearance.
2. Sedimentation volume (F): The sedimentation volume of a suspension is expressed by the ratio of the equilibrium volume of the sediment, Vu to the total volume. Vo of the suspension.
F= Vu/Vo
The value of 1′ for pharmaceutical suspensions lies in between 0 and 1. It determines the physical stability of the suspension. If the value is 1, no sedimentation occurs or there is no clear supernatant. If the value is 0.5. it indicates that 50% of Vo is occupied by sediment and if the value is greater than I. then flocculus is formed.
3. Degree of flocculation ((I): Degree of flocculation is the ratio of the sedimentation volume of the flocculated suspension, F, to the sedimentation volume of the deflocculated suspension, F∞.

When the total volume (Vo) of flocculated suspension is equal to Vo of deflocculated suspension; then, β is (Vu Flocculated/Vu Deflocculated). In simple words, when β is at least 1, it means that the settling amount of the clumped mixture (flocculated) is the same as the settling amount of the separated mixture (Deflocculated). β is a more fundamental parameter than F as it relates the sediment volume of flocculated suspension to the deflocculated system.
4. Rheology: Viscosity of suspension affects and controls the settling of dispersed particles. It also affects the pourability of the formulation. During storage, the viscosity for suspension should be high to prevent sedimentation but at high shear, it is low for ease of administration. Pseudo-plastic substances such as tragacanth, sodium alginate, and sodium carboxymethyl cellulose are used as suspending agents for thixotropic as well as pseudo-plastic types of suspensions. The Brookfield viscometer is used to know the Rheology of the formulation.
The apparatus is mounted on a helipath stand. The ‘T’-bar spindle is made to descend slowly into the suspension, and the dial reading on the viscometer is the measure of the resistance the spindle meets at various levels in sediment. Data obtained for stored samples indicate whether undesired changes took place. This measurement is also made on undisturbed samples of different storage times. The results show how the particles are settling over time. The better mixtures settle more slowly when the spindle turns, meaning the line on the graph stays flat for a longer time. Brookfield viscometer works on the principle, given in the equation below:
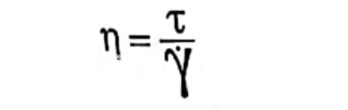
Where η=viscosity in poise is, τ= is the shear stress in dynes / cm2, and γ is the rate of shear (sec-1). A substance that needs a force of 1 dyne per square centimeter to create a flow speed of one inverse second is said to have a thickness of 1 poise (100 centipoises).
5. Zeta potential (0): Zeta potential can be measured through micro electrophoresis apparatus using apparatus such as zeta plus (Brookhaven instruments corporation, USA) or Malvern Zetasizer, Malvern Instruments Ltd, United Kingdom. This demonstrates how steady a spread-out system is. We take around 1 milliliter of a mixture and put it into a plastic container using a dropper. Then, we mix it with clean water. Parameters usually set are the temperature of 25°C and a refractive index (1.33). The zeta potential of suspension is determined on days 0,7,14,21 and day 28 post-formulation in order to assess stability. The flocculated suspension is one in which the zeta potential of a particle is in the range of -20 mV to +20 mV.
6. Particle size measurement: Particle characterization for size and shape is required for the physical stability of the suspension. The way a mixture stays mixed depends on how small the pieces that are spread throughout it. When these pieces change in size as time goes by, we can learn about how well the mixture stays mixed. We can use a special tool called a microscope and another one called a Coulter counter to look at how the size and shape of these pieces change.
7. Crystal growth: In pharmaceutical suspensions crystal growth causes problems in the stability and bioavailability of suspensions. It can reduce this by using a special type of open network mixture (group of particles) that stays together. The particles won’t settle closely together because the mixture is rigid. Polyvinyl pyrrolidone can provide this network and inhibits crystal growth. The rate of crystal growth depends on a variety of parameters such as:
i. Solubility of the drug (i.e. the saturation concentration)
ii. Temperature, temperature difference on storage, and the frequency of temperature cycling.
iii. Degree of super-saturation. iv. Mechanical stress such as stirring.
Advantages of Suspensions
- Pharmaceutical Suspensions are formulated for water-insoluble drugs.
- They mask the bitter or unpleasant taste of the drug.
- Suspension increases drug stability.
- They exhibit controlled or sustained drug release.
- Suspension can improve the chemical stability of certain drugs as in the case of Procaine penicillin.
- A drug in suspension dosage form exhibits a higher rate of bioavailability than other dosage forms such as capsules, compressed tablets, and coated tablets. Duration and onset of action can be controlled through suspension formulation, as in the case of Protamine Zinc-Insulin suspension.
Disadvantages of Suspensions
- Physical stability, sedimentation, and compaction of suspended particles arc observed in suspensions.
- Suspensions are bulky therefore, their handling and transport are a problem.
- They are invariably difficult to formulate.
- An accurate dose is not achieved unless formulated as a unit dosage form.

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
