1.0 OBJECTIVE: To lay down the procedure for the Growth Promotion Test in Microbiology Laboratory.
2.0 SCOPE: This SOP applies to carry out the procedure of Growth promotion test in the Microbiology Laboratory.
3.0 RESPONSIBILITY: 3.1 Executive – to Provide training to new joiners.
3.2 Microbiologist
3.2.1. To perform activity and preparation of SOP.
3.2.2. Keep data as GDP.
4.0 DISTRIBUTION:
Master Copy – Quality Assurance Department
Control copy- Quality Control Department
5.0 PROCEDURE:
5.1 Preparation of Inoculums for Test:
5.1.1 Take a loopful of culture from slant and inoculate in sterile 10 ml saline solutions. Mark this as a stock solution
5.1.2 Take 1.0 ml from stock solution; add in another tube containing 9 ml sterile saline solutions. Mark this as 10-1.
5.1.3 Mark serial dilution up to 10-6.
5.1.4 Mix thoroughly every dilution before transferring it to the test tube.
5.1.5 Pipette 1.0 ml of every dilution from 10-3 to 10-6 tubes into sterile Petri plates in duplicate.
5.1.6 Add approximately 15 to 20 ml of sterile SCDM medium previously cooled to 45°C into Petri plates and mix well. Leave the plate under Laminar airflow for solidification.
5.1.7 Inoculate bacterial culture plate at 32.5 ±2.5°C for two days and yeast and fungal culture at 22.5 ±2.5°C for three to five days.
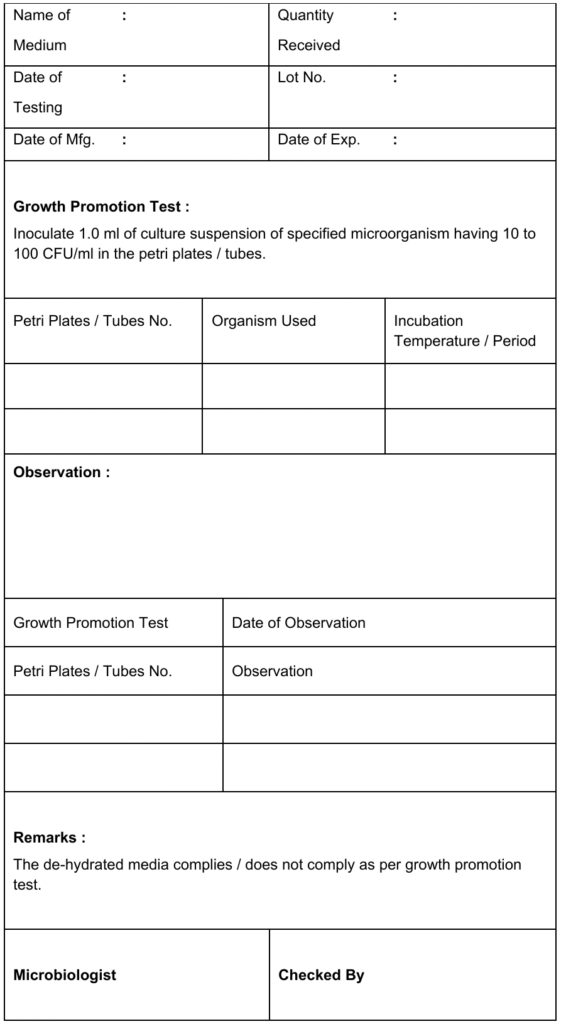
5.1.8 Select the dilution giving 10-to 100 CFU/ml and use the growth promotion test of the medium.
5.2 Testing the medium for growth-promoting ability:
5.2.1 Prepare the required volume of medium
5.2.2 Prepare Petri plates, and tubes as per requirements.
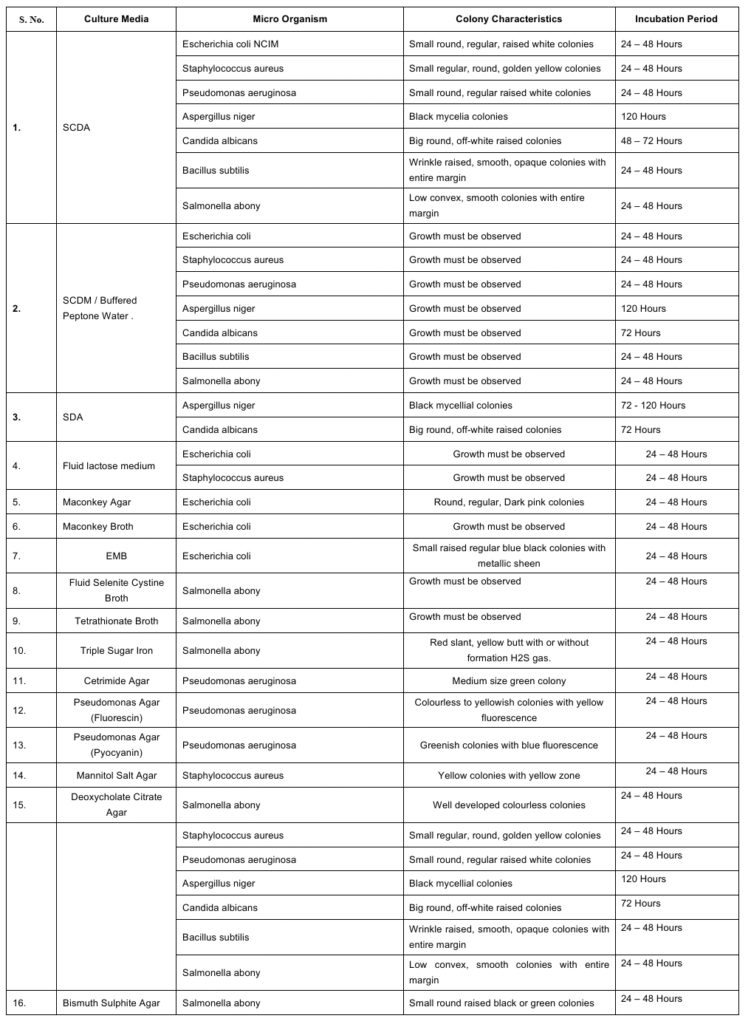
5.2.3 Add 1.0 ml of 10 to 100 CFU/ml dilution of a specific organism to inoculums medium in duplicate, which is mentioned in Annexure–1
5.2.4 Incubate bacterial culture at 32.5 ±2.5°C for two days and yeast and fungal culture at 22.5 ±2.5°C for three to five days.
5.2.5 Observe the growth of culture; if a similar appearance matches USP, BP, and IP standards, then medium passes the growth promotion test.
5.2.6 Record the results in the format of the growth promotion test of the medium as mentioned in Annexure-II.
5.3 Perform the test on receipt of every new lot/batch of medium.
6.0 ABBREVIATION/ DEFENITION:
BP: British Pharmacopoeia
CFU: Colony Forming Unit
DGM: Deputy General Manager
IP: Indian Pharmacopoeia
LAF: Laminar Air Flow
QA: Quality Assurance
QC: Quality Control
SCDM: Soya Bean Casein Digest Medium
SOP: Standard Operating Procedure
USP: United States Pharmacopoeia
BP: British Pharmacopoeia
GDP: Good Document Practices

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
