Standard Operating procedure for estimation of BORON in Pharmaceutical and scientific labs.
Principle:
When Azomethine-H, which comes from condensation of H Acid (8-amino-naptha-1-ol-3.6-disulphonic Acid) and Salicyladehyde, reacts with borate in a watery solution with a pH higher than 6, it a yellow complex that we can measure using a spectrophotometer. To understand the yellow complex We look at how much light it absorbs, especially between 410 nm and 420 nm.
Steps for estimation of Boron:
Materials/ Reagents:
Pure water without boron
Azomethine H Solution: Mix 1.0 gm Azomethine H sodium salt and 3.0 gm L ± Ascorbic acid in a 100 ml bottle, and fill it with water. The solution remains stable when it is stored in a polyethylene bottle for a week at a temperature of 4ºC to 6ºC.
Borate stock: Dissolve 5.719 gm boric acid in 1000 ml water. Keep it in a bottle with a label. Strength 1 ml = 1.0 mg of borate.
Standard Borate: Mix 10 ml stock with 1000 ml water. Keep it in a bottle with a label. (Strength 1 ml = 10µm Borates as B)
Boron Solution: Mix 10 ml stock with 100 ml water. Keep it in a bottle with a label. (Strength 1 ml = 1.0µ Borates as B).
Related: SOP for Estimation of Mercury
Procedure for estimation of BORON:
Make 0.5, 1, 2, 4, and 5 ppm of borate from the standard solution (1 ml = 1 µ of borate). Take 25 ml of each, standard sample and Distilled water as a blank in a 100 ml Volumetric flask. Add 10 ml azomethine H to each. Keep at 20ºC ± 1ºC for 2 hrs in the dark, then make up to 100 ml with water. Measure the highest absorbance.
Calculation:
Boron, mg/L =
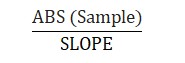
ABS (Spl) – Absorbance of sample
SLOPE – Slope from the standard curve
Reference: IS: 13428 – 1998.”
This method estimates boron levels using specific chemical reactions and measurements.

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
