Preparation and Standardization of 0.01 M Sodium Tetraphenylborate (C6H5)4BNa) solution and calculating formula in Pharmaceutical labs.
Name: 0.01 M Sodium Tetraphenylborate (C6H5)4BNa)
Reagents: Sodium Tetraphenylborate
Sodium Hydroxide
Aluminium Hydroxide
Sodium Chloride
Cetylpyridinium Chloride
Potassium Chloride

Method of Preparation:
- First, mix 3.5 grams of sodium tetraphenylborate in 50 ml of water.
- Shake this with 0.5 grams of Aluminium hydroxide gel for about 20 minutes.
- Then, add 250 ml more water and 16.6 grams of sodium chloride.
- Let it sit for 30 minutes. After that, filter it. Add 600 ml of water and make sure the pH is between 8.0 to 9.0 using 0.1 M sodium hydroxide.
- Finally, dilute it to 1000 ml with water.
Method of Standardization:
- Before using, check its exact concentration in this way:
- Dissolve 7 mg of potassium chloride (previously dried at 150°C for 1 hour) in 5 ml of acetate buffer pH 3.7 and 5 ml of water.
- Add 15 ml of the sodium tetraphenylborate solution and let it stand for 5 minutes.
- Then filter it through a dry, sintered glass filter.
- To 20 ml of the filtrate, add 0.5 ml of bromophenol blue solution.
- Titrate the excess sodium tetraphenylborate with 0.005M cetylpyridinium chloride till the indicator turns blue.
- Repeat this without using potassium chloride.
Calculation:
The calculation formula is :
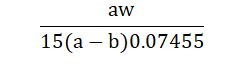
Where ‘a’ is the volume of 0.005 M cetylpyridinium chloride required without potassium chloride, ‘b’ is the volume required with potassium chloride, and ‘w’ is the weight of potassium chloride taken in grams.
Reference: This method is from the Indian Pharmacopoeia.
Read More:
- 0.1 M KOH Solution Preparation and Standardization
- 0.1 M Sodium Nitrite Preparation and Standardization
- 0.05 M EDTA Solution Preparation and Standardization
- Preparation and Standardization of 0.1 M Ceric Ammonium Sulphate
- Preparation and Standardization of 0.1 M Disodium Edetate (EDTA)
- How can I Prepare and Standardize 0.5 M Sulfuric acid?
- 1.0 M Sulfuric Acid Solution- Preparation, Standardization, Reagents, Formula
- 0.1 M Sodium Hydroxide (NaOH), Preparation and Standardization
- Preparation and Standardization of 1 M Sodium Hydroxide Solution (NaOH)
- Preparation and Standardization of 1.0 M Hydrochloric Acid
- Preparation and Standardization of 0.1 N HCl

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
