Standard Operating Procedure for Operating and Validating Anaerobic Systems in Pharmaceutical Microbiology Laboratory.
1.0 Purpose: This document outlines the step-by-step procedure for operating and Validating Anaerobic Systems.
2.0 Scope: This SOP pertains to the operation and validation of the Anaerobic System Mark III (Hi-Media) within the Microbiology Department.
3.0 Responsibilities: Microbiologist: Responsible for implementation of this procedure.
QA Officer / QAM: Review and overall compliance with this Procedure.
4.0 Procedure for Validating Anaerobic Systems:
4.1 Cleaning and Preparation:
4.1.1 Ensure the instrument is clean and free from dust.
4.1.2 Place vented disposable plastic Petri plates into the carrier to facilitate gas transfer between the interior and exterior of the plates.
4.2 Inserting Anaero Indicator Tablet:
4.2.1 Open the sachet of Hi Media’s Anaero Indicator Tablet (LE001B) and remove one tablet pack.
4.2.2 Insert the pack into the upper clip on the plate carrier.
4.3 Assembling the System:
4.3.1 Lower the plate carrier into the Polycarbonate base.
4.3.2 Read the instructions on the Anaero Gas Pack carefully.
4.3.3 Open one Anaero Gas Pack (LE002A-LE002F) as indicated by the cut mark.
4.3.4 Place the sachet into the lower clip of the plate carrier.
Related SOP: SOP for Operating and Validating a Biosafety Cabinet
4.4 Securing the System:
4.4.1 Place the lid on the base, ensuring the O-ring is correctly positioned against the flange for a secure fit.
4.4.2 Apply the beam clamp and tighten the knurled wheel.
4.5 Monitoring Anaerobic Condition:
4.5.1 The Anaerobic Indicator tablet should remain pink, indicating anaerobiosis. Any leakage causing aerobic conditions will turn the tablet purple.
4.6 Completion and Disposal:
4.6.1 After incubation, discard the Anaerobic Indicator tablet with normal laboratory waste.
4.6.2 Carefully remove the exhausted Anaero Gas Pack sachet following the instructions on the cover.
4.7 Lid and Jar Maintenance:
4.7.1 Keep the interior of the lid and jar dust-free.
4.7.2 Utilize provided plastic dust caps to prevent dust particles from interfering with the valve core’s operation.
5.0 Routine Maintenance:
5.1 Cleaning:
5.1.1 Clean the Anaerobic jar by swabbing with 70% alcohol.
5.1.2 For enhanced disinfection, add 20ml of 10% formalin solution and incubate the anaerobic system at 37°C overnight.
5.1.3 Avoid using sodium hypochlorite or strong detergent solutions.
5.2 Lid Care:
5.2.1 After each use, clean and dry the lid using soft tissue, being cautious not to scratch the surface coating.
5.2.2 Store the jars open and inverted, in a warm dry place, to prevent contamination by dust.
5.3 O-ring Inspection:
5.3.1 Regularly inspect the O-ring to ensure it is undamaged. Replace if any signs of splitting or faults are observed.
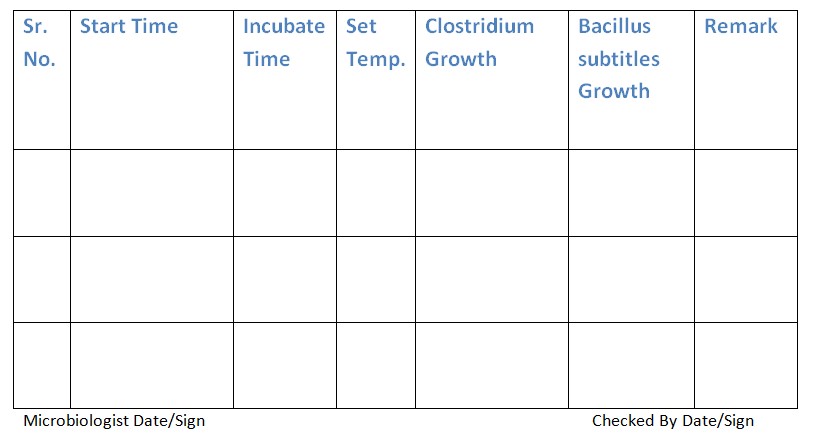
6.0 Validation:
6.1 Place Petri dishes lined with Bacillus subtilis and Clostridium cultures in the carrier.
6.2 Seal the anaerobic jar and incubate the assembly at 37°C for 24-48 hours.
6.3 Record the growth.
6.4 Clostridium bacteria will exhibit significant growth, while Bacillus subtilis growth will be inhibited due to the lack of oxygen.
Related SOP: SOP on Operation and Calibration of the Antibiotic Zone Reader
7.0 Abbreviations:
SOP: Standard Operating procedure
QA: Quality Assurance
%: Percentage
°: Degree
ml: Mililiter
8.0 Documentation:
Refer to Annexure – 1 for the Anaerobic System usage log book.
Refer to Annexure – 2 for the Validation of Anaerobic System.
| Date and Time | Instruments Name/ID | Start Time | Performed By | End Time | Checked By | Remark |

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
