1.0 Objective: To lay down the procedure for Pest & Rodent Control within the manufacturing areas.
2.0 Scope: This pest & rodent control standard operating procedure within the manufacturing plant, factory areas and food industries.
3.0 Responsibility: The housekeeping department officer/Executives is responsible for the implementation of this procedure.
4.0 Accountability:
QA Head shall be accountable for the implementation of this SOP.
5.0 Procedure for Pest & Rodent Control
5.1 Pest Control
5.1.1 Pest control activities shall be executed by hiring outside pest control agencies like; Rentokil PCI.
5.1.2 Companies hire outside pest control agencies by Annual contract.
5.1.3 The pest control activity in the control area shall be performed as per the procedure or weekly.
5.1.4 Only the contractor’s well-trained person shall be permitted for conducting the pest control activity.
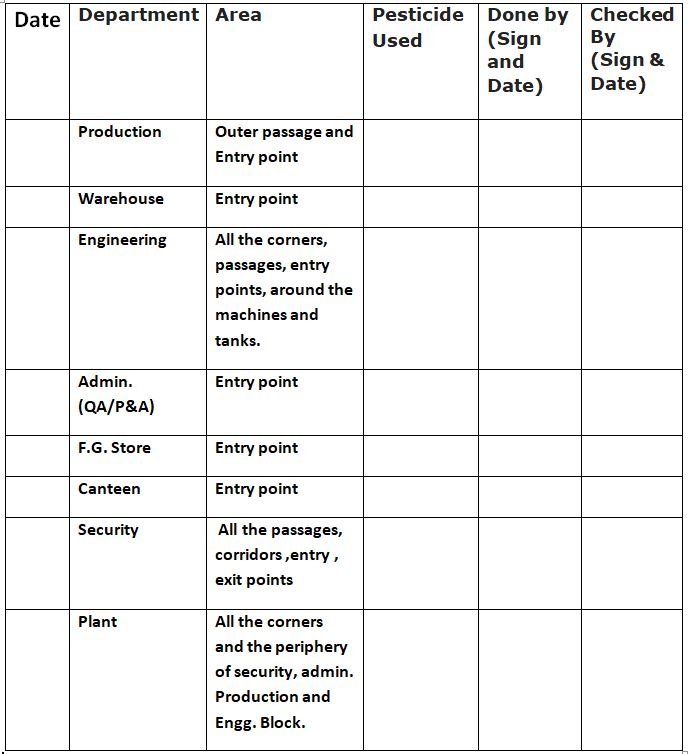
5.1.5 The control area and the pesticide points shall be cited as per Annexure-I i.e. ‘Pest Control Records’.
5.1.6 The person, who shall be involved in pest & rodent control activity, shall be well trained and must wear a proper gown (as per gowning SOP), nose mask, hand gloves & shoe cover.
5.1.7 Further services must be requested from the contractor and a record of them kept if there is a seasonal impact on the environment or climatic change that leads to an increase in insects, flies, etc.
5.1.8 Record shall be checked by the housekeeping supervisor.
5.1.9 Pesticide storage is not allowed on factory sites.
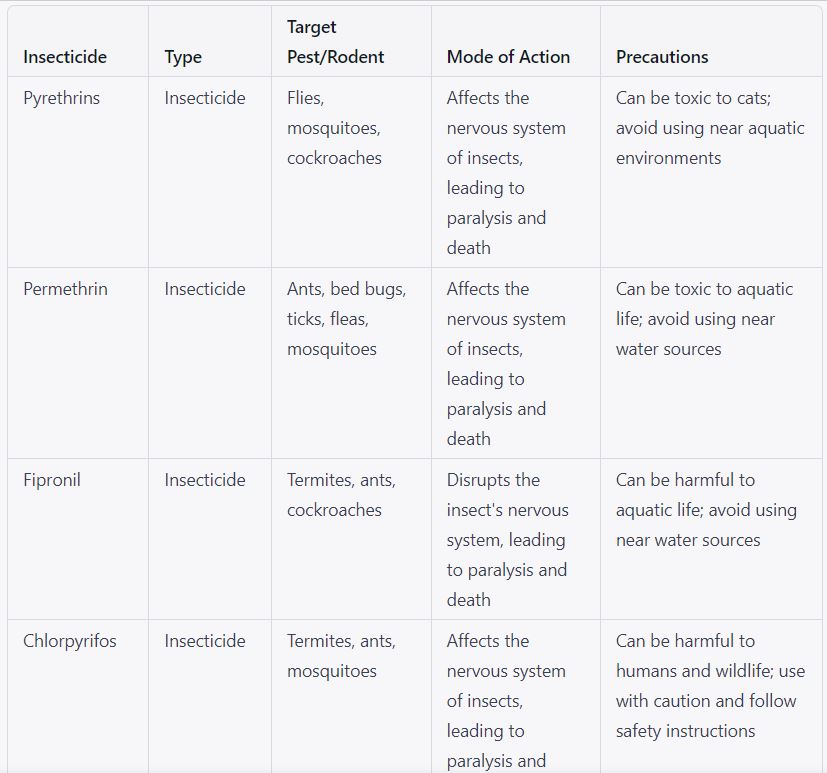
5.1.10 Only permitted pesticides shall be allowed in controlled areas within the premises as per annexure-III.
5.1.11 While purchasing pesticides, a contractor must also check the material safety data sheet and ensure that the pesticides are reliable.
5.1.12 Pesticide containers must be distributed, prepared, used, and disposed of safely by the contractor’s staff at their location.
5.1.13 Before and after the pest treatment, make sure the area is free of medication-related components until housekeeping cleans it and QA approves it for usage.
5.1.14 Make sure that all pest management work is completed in accordance with Annexure-I, and after QA Department evaluation.
5.2 Rodents Control
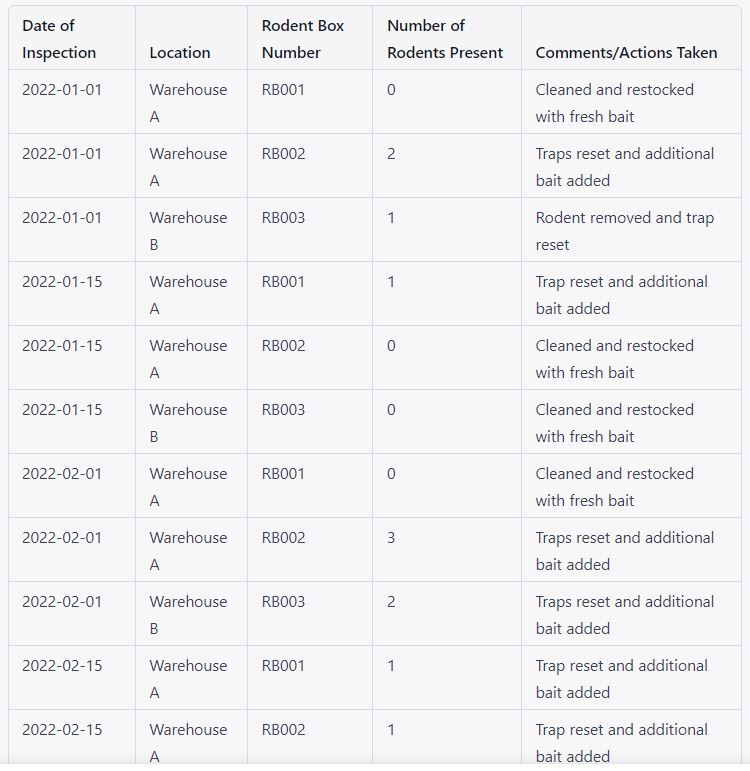
5.2.1 The “Glue pad” must be placed inside the rodent box by the contractor’s skilled staff every two weeks in order to control the rodents, and a record must be kept as per Annexure-II.
5.2.2 The roda boxes shall be identified by a unique number on the basis of their location e.g. RB001
5.2.3 Where R stands for rodent
B stands for Box
001 stands for serial number.
5.2.3 Roda boxes location & ID no. shall be as per Annexure-II.
5.2.4 Under the supervision of the instructor, housekeeping staff must verify roda boxes inside the premises twice a week. Records of the inspections must be kept in accordance with Annexure II.
5.2.5 During inspection if any rodents shall be found then housekeeping personnel shall clean the roda boxes and replace them with another glue trouble gum pad.
5.2.6 Rodent shall suppress and records shall maintain in Annexure-II
5.3 Insects Control
5.3.1 Insectocutor devices must be put wherever required and at each entry point to control insects.
5.3.2 Every insectocutor must have a special identifying number, such as IS01, where IS stands for the insectocutor and 01 for the sequential serial number.
5.3.3 According to Annexure-V, a list of insectocutors must be maintained.
5.3.4 Monitoring of the insectocutor must be done twice daily, and records must be kept in accordance with that.
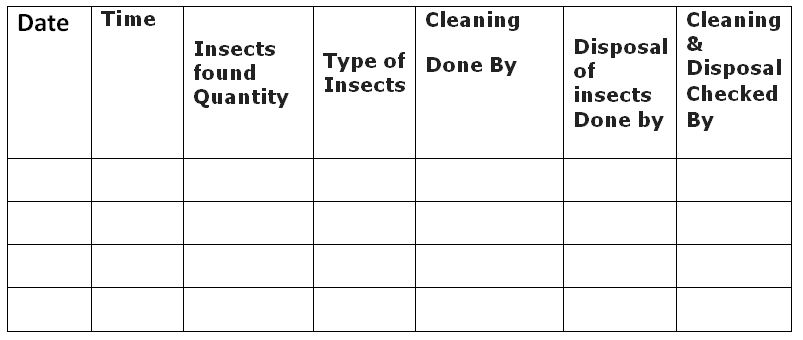
5.3.5 Cleaning of insectocutors shall be done daily.
5.3.6 The number of insects and the type (which will define the insecticide or insecticides) must be recorded daily, and a record must be kept of each.
5.3.7 Daily cleaning of the insecticide tray, disposal of the killed insects, and maintenance of records in accordance with Annexure-IV are required.
5.4 Safety Precautions
5.4.1 Avoid contact with the skin and eyes because the insecticides and pesticides used are extremely harmful.
5.4.2 While spraying, inhalation should be avoided.
5.4.3 Oral ingestion to be avoided.
5.4.4 In case of poisoning, call the emergency immediately.
6.0 Abbreviations and Definitions
SOP: Standard Operating Procedure
No. : Number
GMP: Good Manufacturing Practices
QA: Quality Assurance
ID No. : Identification No.
E.g.: Example
& : And
i.e.: That is
7.0 Forms and Records (Annexures)
|Pest control record – Annexure-I
Rodent box inspection record – Annexure-II
Chemicals to be used for pest & rodent control – Annexure-III
Insectocutor log record – Annexure-IV
List of insectocutors – Annexure-V
8.0 Distribution
Master copy sop for pest control – Quality Assurance
Controlled copies sop for pest control – Quality Assurance, Production, warehouse, Quality Control, Engineering, and Human Resources.
‘Pest Control Records’ Annexure-1
Rodent box inspection record – Annexure-II
Chemicals to be used for pest & rodent control – Annexure-III
Insectocutor log record – Annexure-IV
List of insectocutors – Annexure-V

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
