Standard Operating Procedure on Procurement, Storage, Usage, and Destruction of Reference Standards and Impurities. In this SOP you will learn about the following:
- SOP on Reference Standards and Impurities
- Procedure for Reference Standards and Impurities
- Storage of Reference Standards / Impurities
- Usage of Reference Standards / Impurities
- Precautions
- Destruction of Reference Standards / Impurities
- SOP on Reference Standards and Impurities -Annexures

1.0 Objective: 1.1 To lay down a procedure for the Procurement, Storage, Usage, and Destruction of Reference Standards and impurities.
2.0 Scope: 2.1 This procedure is applicable for the Procurement, Storage, Usage, and Destruction of Reference Standards being used in the Quality Control Department.
3.0 Responsibility:
3.1 QC Officer/ Executive shall be responsible for proper usage of documents of Reference
Standards.
3.2 The QC section in-charge shall be responsible for the availability and procurement of Reference Standards.
4.0 Accountability:
4.1 QC Head or his / her Designee shall be accountable for compliance with this SOP.
5.0 Procedure for Reference Standards and Impurities:
5.1 Reference Standards:
5.1.1 Reference Standards are highly characterized specimens of drug substances, impurities,
degradation products, dietary supplements, compendial reagents, and performance calibrators.
5.1.2 Reference standards should be prepared and controlled following written procedures.
5.1.3. Reference standards should be established as suitable for their intended use. Their qualification and certification, as such, should be clearly stated and documented.
5.1.4. Whenever compendial reference standards from an officially recognized source exist, these
should preferably be used as primary reference standards unless fully justified (the use of secondary standards is permitted once their traceability to primary standards has been
demonstrated and is documented).
5.1.5 These compendial materials should be used for the purpose described in the appropriate
monograph unless otherwise authorized by the National Competent Authority.
5.1.6 Reference standards should be marked with the preparation and opening date and the signature of the person who prepared them.
5.1.7. Where necessary, the date of receipt of reference standards should be indicated on the
container. Instructions for use and storage should be followed. In certain cases, it may be
necessary to carry out an identification test and/or other testing of reagent materials upon
receipt or before use.
5.1.8 Reference Standards are employed in the Identification, Purity testing, and Assay of substances for Pharmaceutical use and Pharmaceutical Preparations. They are not intended for use as drugs.
5.1.9 Reference Standard is a primary standard that has the appropriate quality within a specified
context and is accepted without requiring comparison to another substance.
5.2 Impurities:
5.2.1 Impurity is defined as any substance coexisting with the original drug, such as starting material or intermediates, or that is formed, due to any side reactions.
5.3 Indian Pharmacopoeia Reference Standards (IPRS):
5.3.1 Indian Pharmacopoeia Reference Standards, abbreviated to IPRS, are issued/ certified by the
Indian Pharmacopoeia Commission (IPC) or by the Laboratories notified by IPC.
5.4 British Pharmacopoeia Reference Standards (BPRS):
5.4.1 The British Pharmacopoeia Laboratory is responsible for the establishment and maintenance of British Pharmacopoeia Chemical Reference Substances (BPCRS), to support the monographs
of the BP.
5.4.2 The respective BPCRS can be obtained directly from the British Pharmacopoeia Commission
(BPC) or from the respective authorized agencies/bodies.
5.4.3. The current batch number of reference standards is always listed on the BP Site.
5.5 European Pharmacopoeia of Reference Standards (EPRS):
5.5.1 EP Reference Standards / Impurities, Information on availability and the status of the current Batch / Lot Number is provided in the Reference Standards Catalogue available from the European
Directorate for the Quality of Medicines (EDQM) and its EP Site.
5.5.2 The EP Reference Standards Catalogue is updated three times a year and is available in
English only. It includes the list of all the reference Substances currently available together with
instructions for use.
5.6 United States Pharmacopeia Reference Standards (USPRS):
5.6.1 USP Reference Standards / Impurities, Information on availability and the status of the current Batch / Lot Number is provided in the Reference Standards in USP.
Related SOP: SOP on Receipt and Handling of Laboratory Samples
5.7 Procurement of Reference Standards and Impurities:
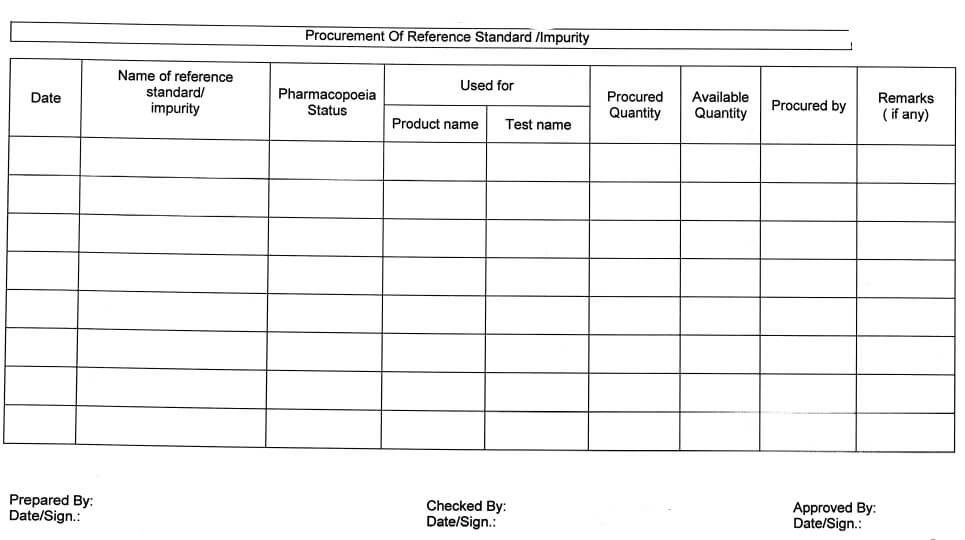
5.7.1 After the receipt of information of launching of a new product or existing lot of standards is
expired (Validity period over) or a new lot has been issued/released, by the respective pharmacopoeia commission/convention, procurement procedure for the respective standards/ impurities shall be initiated by the section head/designee-QC.
5.7.2 Reference standards/Impurities/are required and to be procured/arranged for the analytical
purpose under the following situations:
5.7.3. The Product is Pharmacopoeial and the Pharmacopoeial Reference Standard/Impurity is
available.
5.7.4. The Product is Non-Pharmacopoeial and the Pharmacopoeial Reference Standard/Impurity is
available.
5.7.5 In the above cases, the Section Head/Designee—QC shall raise the indent and arrange for the
Pharmacopoeial Reference Standards and Impurities.
5.7.6 Reference standards shall not be used after the validity date mentioned in the catalog/ site or as per the respective specified validity period.
5.7.7 The validity status of the Pharmacopoeial Reference Standard/ Impurity shall be checked by
Executive/Officer or above monthly & print out shall be taken for the applicable reference
standard / impurities and the same shall be filed for verification. or before its uses, against the
respective Catalogue(s) for the availability of the current Lot/Batch No. as per
annexure -ll.
5.7.8 After reviewing the Reference standard/impurities shall be segregated which is going to be
expired on next month & keep separately.
5.7.9 The expired reference standards/Impurities shall be removed from the stock & will not be used for further analysis.
5.7.10 The receiving status of reference standards/Impurities should be verified monthly as per Annexure-V(Procurement of new or expired reference standard/impurity).
5.7.11 If the product is/ are not Pharmacopoeial or reference standard/ impurities not available in any pharmacopeia, in such cases the reference standards/impurities shall be arranged from the
Authentic chemical manufacturers (e.g. Fluka, Merck, Lancaster, Sigma Aldrich, JT Baker,
Rankemetc.), other agencies, or can be arranged from the respective approved vendor/supplier.
A certificate of analysis for the same shall be available in the QC department.
5.7.12 All the standards/Impurities, received from authentic/authorized/certified/recognized sources, Pharmacopoeial agencies, and approved vendors shall be considered as Primary
Standard/Impurity.
5.8 On receipt, the Reference Standard/impurity, Vial/Container shall be checked for
following attributes (But not limited to):
5.8.1 Integrity of the Vial/ Container.
5.8.2 Pharmacopoeial Status.
5.8.3. Label of the respective Vial/ Container.
5.8.4 Name of Reference Standard’ Impurity.
5.8.5 Catalog No. / Lot No. / Batch No. of Reference Standard/ Impurity.
5.8.6 Availability of respective COA, MSDS, etc.
5.8.7 Other information like potency, instruction of usage, and storage, etc.
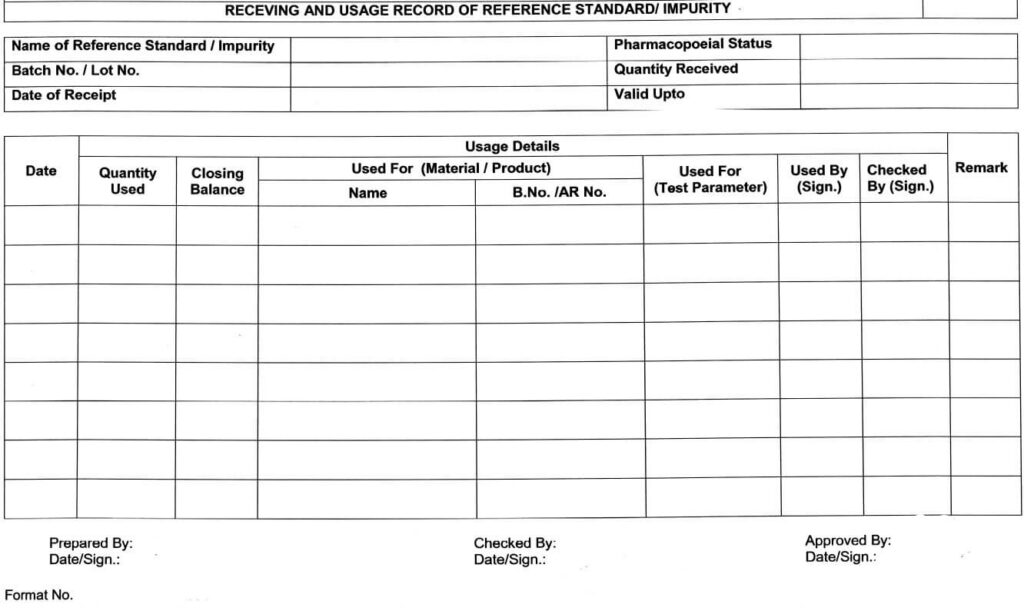
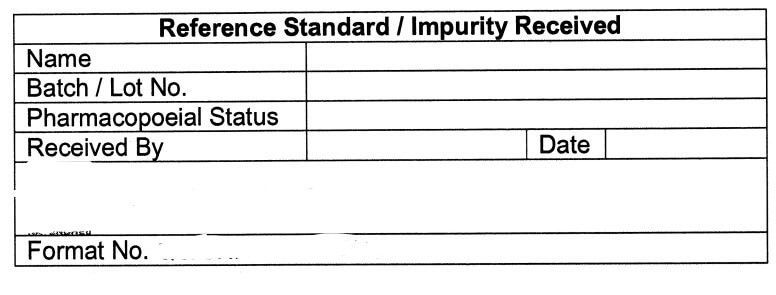
5.9 Receipt record of all Reference standard(s)/Impurities, such as name, Lot No., Date of receipt,
Received by, Quantity received, etc. shall be maintained as per Annexure-|, and same shall be
updated as and when applicable by Executive/Officer QC.
Related SOP: SOP for Glassware Cleaning in QC Lab
5.10 Usage of Reference Standards / Impurities:
5.10.1 The official and authorized uses of Reference Standards are specified in the respective
monographs and general chapters, and they include (But Not Limited to):
5.10.2 Use in Quantitative analysis such as Assays for Drug Substances and Formulations (Drug
Products), Limit Tests, Preparation of working standards, etc.
5.10.3 Use in Qualitative analysis such as Identification tests, System Suitability Tests, etc.
5.10.4 Use as Performance Standards and Calibrators, such as Dissolution Calibrators, Melting Point
Standards, Verification of Particle count, etc.
5.10.5 Reference Standards do not carry an expiration date as long as they are in distribution. A lot of any RS may be used as long as it is listed as “Current Lot” in the current (most recent) official
Reference Standards Catalogue.
5.10.6 Users are responsible for ensuring that the Reference Standards/Impurities they are using have official status either as a “Current lot” or as a “Previous lot” within the valid use by date. The same can be verified by referring to the information available with reference standard catalog.
5.10.7 Directions for usage are available on the label of each Reference Standard. These are a lot-
specific. The label includes safety warnings, required information for controlled substances, and
calculation values for standards with quantitative applications.
5.10.8 As and when required, an appropriate quantity of the Reference standard shall be used for the qualification of the Secondary Standard (Working Standard) and for analysis of product /materials in case the working standard is not available.
5.10.9 The potency mentioned on the label or COA shall be used for calculation. If potency is not
mentioned on the label or COA, 100.0 % Potency (on an is basis) shall be considered for all
calculations.
5.10.10 The reference standard shall be tested for water content in any case at the time of use if the monogram of the pharmacopeia states the water content.
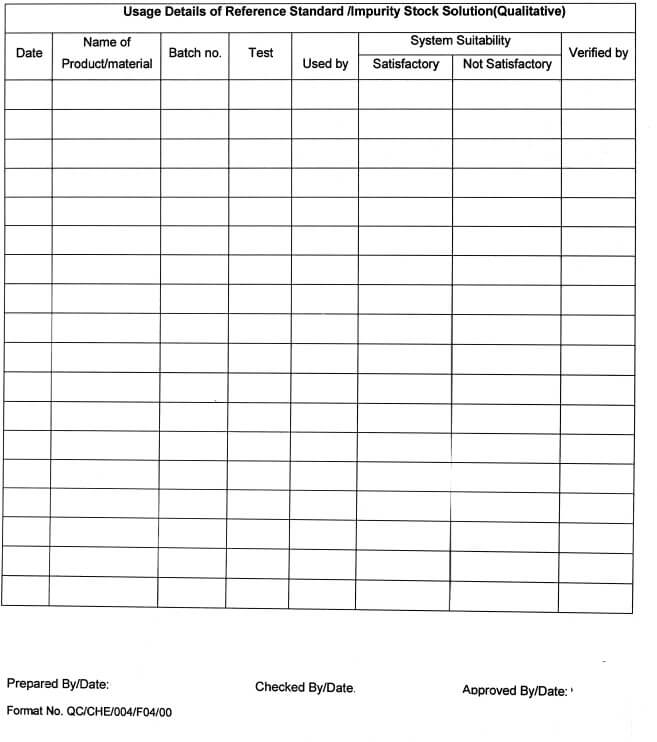
5.10.11 After every use, the consumption record of the quantity of the respective Standard / Impurity shall be maintained in Annexure-l.
5.10.12 The quantity of the respective Reference standard / Impurity shall be used for a particular test and can be reduced as per the quantity available in the respective vial / Container. While doing so, prior approval shall be taken from the Head/Designee-QC and the final concentration of the
the solution shall remain unchanged.
5.10.13 Where a Reference Standard is required to be dried at a specific temperature before being used; dry only the appropriate quantity of material and not the original vial /container.
5.10.14 If the quantity received/remaining of a particular standard/impurity is less for qualitative or
quantitative use, then the dilution (The Stock of the Final Solution) being prepared can be stored in
refrigerator or at temperature as per the COA (if available) of that particular standard/ impurity
and labeled as per Annexure-lll & Annexure-lV, use_ it till all the respective System Suitability
parameters are met as per the specified criteria and no other extra peak(s) Interfering with the
respective analysis are observed during the analysis or up to one year.
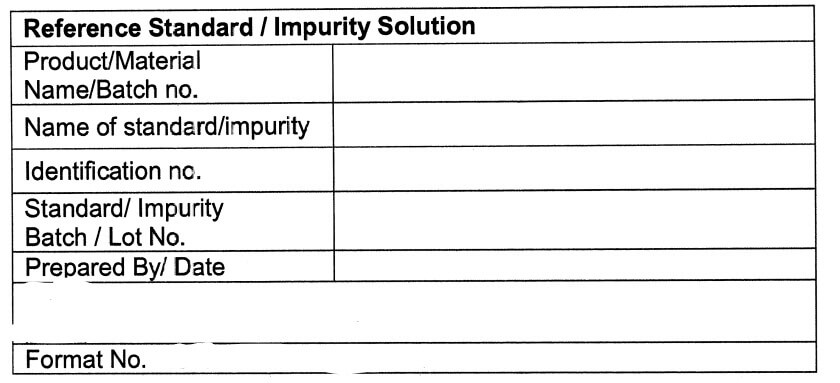
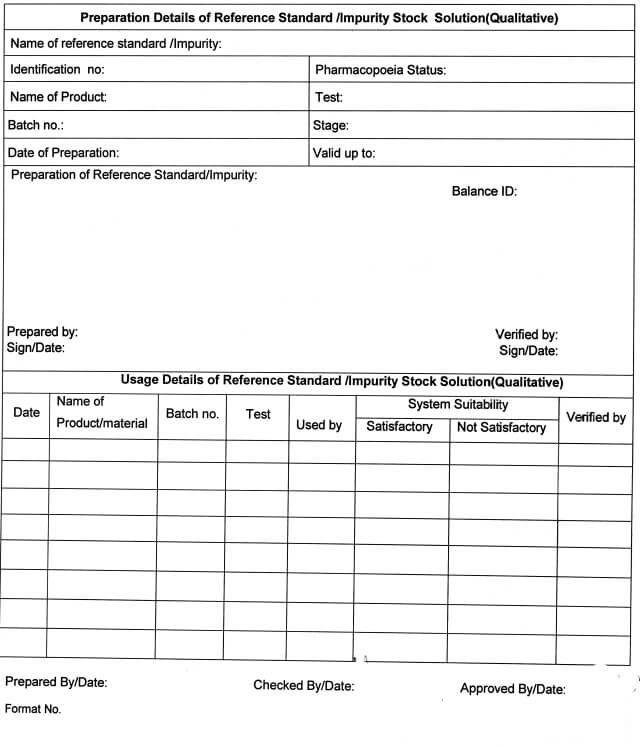
5.10.15 Fill the details regarding the Preparation of Reference Standard /impurity Stock Solution
(Qualitative) in Annexure -VI and Preparation of Reference Standard /Impurity Stock Solution
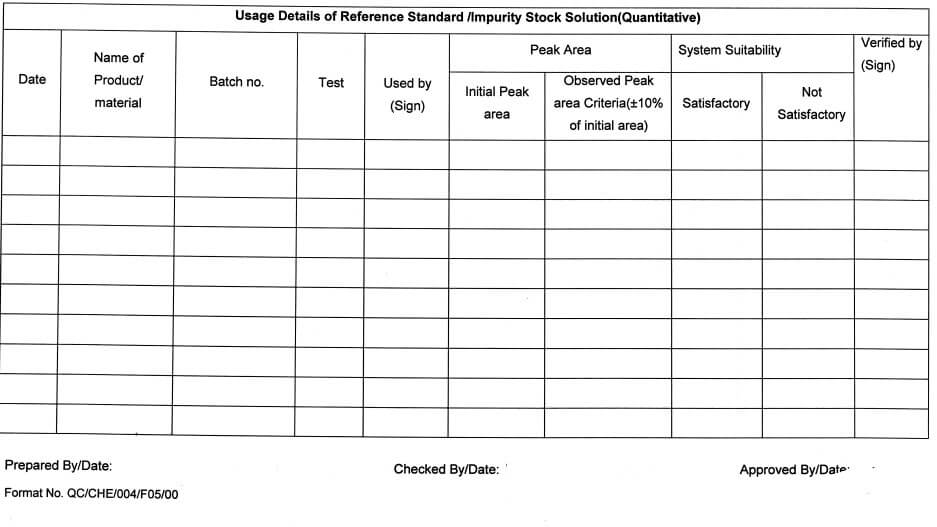
(Quantitative) in Annexure -VII.
5.10.16 A unique identification number will be given to the final stock solution prepared for system suitability as:
SST/XY/001/00
Where.
SST = System Suitability
XY = Year of Preparation
001 = Serial no.
00 = Version no.
5.10.17 All such solutions shall be used for qualitative purposes (Identification test, System suitability, etc.) For quantitative purposes (if required) the traceability of the stock solution shall be ensured & approval of the QC Head shall be taken.
5.10.18 Reference Standards and Impurities shall be accurately weighed, taking into account the
relatively large errors associated with weighing of small quantities.
5.11 Storage of Reference Standards / Impurities:
5.11.1. After usage, all reference standard/impurity vials /containers shall be closed properly (with
rubber stopper and aluminum seal) and stored at their specified place and under specified
conditions.
5.11.2 Each reference standard shall be preserved in its original vials/containers under lock and key
5.11.3 Extra care/precautions shall be taken while handling any hygroscopic/Photosensitive Reference standards/ Impurities.
5.12 Destruction of Reference Standards / Impurities:
5.12.1 Reference standard/impurity shall be destroyed as per the following conditions (But rot limited to) and the record shall be maintained as per the format in Annexure-l, in the remark column.
5.12.2 After the expiration (Validity Over) of existing Lot/ Batch No.
5.12.3 Existing Batch / Lot No. gets decomposed/contaminated.
5.13 Precautions:
5.13.1 During Usage, always transfer only the required quantity from the vial/container.
5.13.2 Never transfer the remaining quantity of the reference standard back to the original vial /
container.
5.13.3 If the respective Standard is stored at 2 to 8°C, never use it immediately after taking it out from the refrigerator. Allow it to attain the ambient temperature before use.
5.13.4 Never insert any external device, such as a spatula, glass rods, butter paper, etc. into the vials, as it may contaminate the reference standards.
5.13.5 Read proper instructions before using Reference standards/impurities as per MSDS.
6.0 Reference:
As per GMP Guideline/In house.
7.0 Annexures / Attachments:
Receiving/usage Record of Reference Standard/ Impurity (Annexure-I)
Validity Status for Reference Standards/Impurities (Annexure-II)
Specimen Label for Reference Standard / Impurity Solution (Annexure-IIl)
Specimen Label for Reference Standard / Impurity Received (Annexure-lV)
Procurement of reference standard/ impurity (Annexure-V)
Preparation /consumption of reference standard/impurity (Qualitative) (Annexure-VI)
Preparation /consumption of reference standard/impurity (Quantitative) (Annexure-VII)
8.0 Distribution:
Master Copy: QA Department.
Controlled Copy: QC Department.
Display Copy: Chemical Analysis Lab.
9.0 Abbreviations:
SOP: Standard Operating Procedure
QC: Quality Control
IP: Indian Pharmacopoeia
BP: British Pharmacopeia
EP: European Pharmacopeia
USP: United State Pharmacopeia
MSDS: Material Safety Data Sheet
COA: Certificate of Analysis


Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
