1.0 Purpose: To lay down the procedure for Washing, sterilization of clean room Garments
2.0 Aim: This Standard Operating Procedure is applicable to the Washing, sterilization of clean room Garments in pharmaceuticals.
3.0 Responsibility: 3.1 The line operator in operation processes shall carry out the operation process of Washing, and sterilization of clean room Garments.
3.2 officers/seniors/executives shall supervise the process.
3.3 Production Manager shall be responsible for the implementation of SOP.
3.4 Head –Quality Assurance /designee shall be responsible for compliance with SOP.
4.0 Procedure:
4.1 Washing of core area garments:
4.1.1 Ensure that the garment washing machine and garment drying machine are working properly.
4.1.2 Ensure the availability of purified water and cleaning agent.
4.1.3 Do not overload the washing chamber of the machine.
4.1.4 Collect all garments from the de-gowning area Room and bring them to the dirty laundry Room in a crate.
4.1.5 Transfer the dresses to the washing chamber of the garment washing machine.
4.1.6 Each quantity of dress and hood should not exceed more than its capacity.
4.1.7 Booty and foot cover load should not exceed more than its capacity.
4.1.8 Start the washing cycle.
4.1.9 After completion of the washing cycle transfer the garments to the drying chamber for drying.
4.2. Drying of core area garments:
4.2.1 Transfer the garments to the drum of the drying machine.
4.2.2 After completion of the drying cycle transfer the garments under a Laminar air flow hood located in the Laundry Room for packing in sterile pouches.
4.3 Packing and sealing of core area garments:
4.3.1 Now fold the dresses as per the folding procedure.
4.3.2 See the photograph to fold the dress provided below.
4.3.3 After folding of garments pack those in a sterilization packing reel.
4.3.4 Pack the garments with the help of a pouch-sealing machine.
4.3.5 Mark the sterile pouch with dress sizes XL, M, and S.
4.3.6 Follow the pictorial instructions for the packing and sealing of dresses provided in Annexure 2.
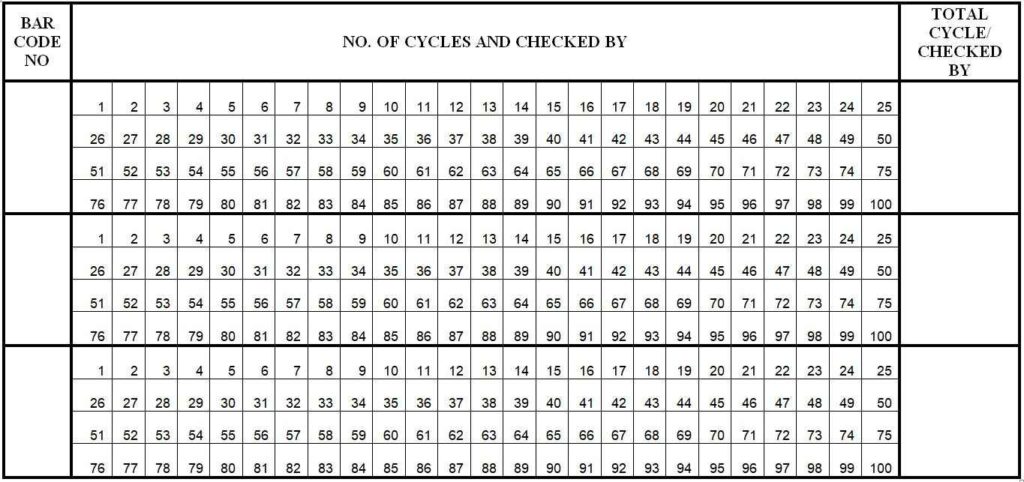
4.3.7 Check the 8-digit bar code provided on the collar of the dress.
4.3.8 Enter the bar code in the bar code log book to identify the No. of sterilization cycles for each dress.
4.3.9 Fill the log book of the bar code as annexure below.
4.4 Sterilization of core area garments:
4.4.1 Sterilize the garments load in an autoclave with validated cycle time and temperature.
4.4.2 Follow SOP on “packing of materials for sterilization” for the sterilization of the garments.
4.5 Recheck/ frequency of inspection of garments:
4.5.1 Verify the no. of sterilization cycle of dresses from the log book of the bar code.
4.5.2 Make a regular check for the fiber of the dress whether it’s in good condition to wear inside the clean room or not.
4.5.3 The garments are torn out, shedding fibers, without buttons and tying string. Such dresses should be sent for repair or if not able to repair should be discarded.
4.5.4 The core area garments can withstand a minimum of hundred sterilization cycles.
4.6 Washing, Drying, Packing, Sealing, and Sterilization of Boiler Suit:
4.6.1 Repeat step no. 4.1.1 to 4.1.3.
4.6.2 Collect the white boiler suits from the de-gowning area room and bring them to the dirty laundry room in a crate.
4.6.3 Transfer the garments to the washing chamber and start the washing cycle.
4.6.4 After completion of the washing cycle transfer the garments to the drying chamber for drying.
4.6.5 Transfer the garments to the drum of the drying machine.
4.6.6 After completion of the drying cycle transfer the dresses under a Laminar air flow hood located in the Laundry Room for packing in sterile pouches.
4.6.7 Pack the boiler suits in sterile pouches as shown below:



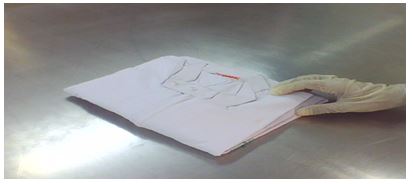


4.6.8 Seal the packed dress in the sealing machine & transfer it to the material sterilization area
4.6.9 Follow SOP “packing of materials for sterilization” for the sterilization of the garments.
4.6.10 The white boiler suit (for preparation areas like a solution, disinfectant, material, laundry, and vial washing) which are torn out, shedding fibers, without buttons and tying string. Such garments should be sent for repair or if not able to repair should be discarded.
5.0 Abbreviations:
No. : Number
QA: Quality Assurance
SOP: Standard Operating Procedure
mL: Millie liter
XL: Extra large
M: Medium
S: Small

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
