Most pharmaceutical manufacturing companies have realized that in order to achieve the goal of long-term business survival in today’s regulated environment, they need to install a full eMES system, also known as the eBMR system. However, the current infrastructure situation prevents such an implementation from proceeding. Before discussing the difference between BMR/BPR and eBMR, it is important to understand their distinctions.
Pharmaceutical companies operate in one of the most dynamic and regulated industries all over worldwide. The frequency of regulatory audits has increased, along with the number of warning letters and import restrictions. As a result, many pharmaceuticals struggle to pass regulatory audits, which can lead to loss of revenue and significant costs associated with re-audit success.
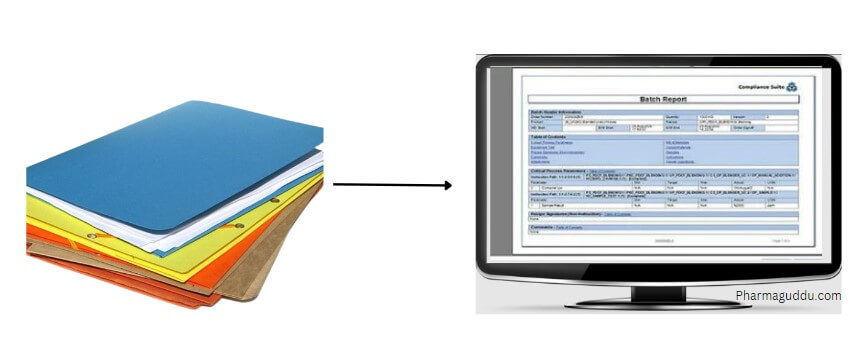
Difference between BMR And eBMR/eBPR
BMR: A batch manufacturing record is a written document that is used to record the entire manufacturing process and history of that product. in other words, it tells you how to manufacture a product or batch and records the way that happens.
BPR/BPCR: A batch packing record is a written document starting from dispensing of packing materials to the end of the dispatch stage, which tells about the procedure and stepwise instructions to be followed during the packing.
eBMR: eBMR refer to electronic batch manufacturing record. It is a web-based solution that streamlines the manufacturing process from dispensing of raw materials to manufacturing and packaging. The eBMR system is very helpful in records data from different stages, making it easier to track and manage the manufacturing process. With this system, manufacturers can quickly and accurately record information about each batch of products they produce. By using eBMR, manufacturers can reduce the risk of errors and improve the overall efficiency of their manufacturing operations.
Software for eBMR/eBPR:
- SoftBMR
- Atachi system
- Serjen system
- Laurus MES software
Advantages of eBMR in Pharmaceuticals
- eBMR complies with 21CFR Part-11 of the FDA and EU Annex-11
- Users shall be able to do manual entry in the centralized application by which we shall get data, users, workflow, reports, and status.
- To provide application-based reading and writing provisions and controls in order to avoid recording errors.
- To meet documentation requirements as per ALCOA+.
- To Facilitate faster getting or retrieval of historical data.
- Minimization of paper uses.
- To capture real data and real-time by the right person in batch process control record.
- BMR And eBMR both enforce compliance, but eBMR minimizes the review cycle time, hence also decreasing the manufacturing cost.
- It helps in the process and product tracking.
- Improve the visibility of the manufacturing process.

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].

eBMR & eBPR is usefull ratherthan current scennerio [BMR/BPR].