Standard Operating Procedure for Acceptable Quality Level (AQL) includes; Purpose, Scope, Responsibility, Precautions, Procedure, Classification of Defects for AQL, and AQL Charts.
1.0 Purpose: To lay down the standard procedure for an acceptable quality level for semi-finished tablets to evaluate physical attributes before packing.
2.0 Scope: This sop is applicable to all tablet products manufactured at the plant.
3.0 Responsibility:
3.1 The concerned QA officer and production officer shall be responsible for sampling.
3.2 The concerned production officer shall be responsible for raising requisition formate for AQL.
3.3 IPQA officer shall be responsible for performing the Acceptable quality level (AQL).
3.4 Section head production shall be responsible for checking, and section head IPQA/Designee shall be responsible for authorizing the same.
3.5 Head production and Head QA shall be responsible for compliance with this SOP.

4.0 Precautions:
Wear hand gloves and a nose mask before sampling and while performing Acceptable quality level (AQL).
5.0 Procedure for Acceptance Quality Level (AQL):
5.1 For Uncoated Tablets: after completion of compression, make the necessary entries in AQL requisition format “acceptance quality level for semi-finished (tablets)” and hand over the same to the IPQA officer.
For Coated tablets: after completion of coating, 100% inspection shall be carried out of coated tablets. Weighing records shall be filled in “visually inspected uncoated/coated tablets weighing records” to make the necessary entry in Acceptable quality level AQL format “acceptance quality level for semi-finished (tablets).”
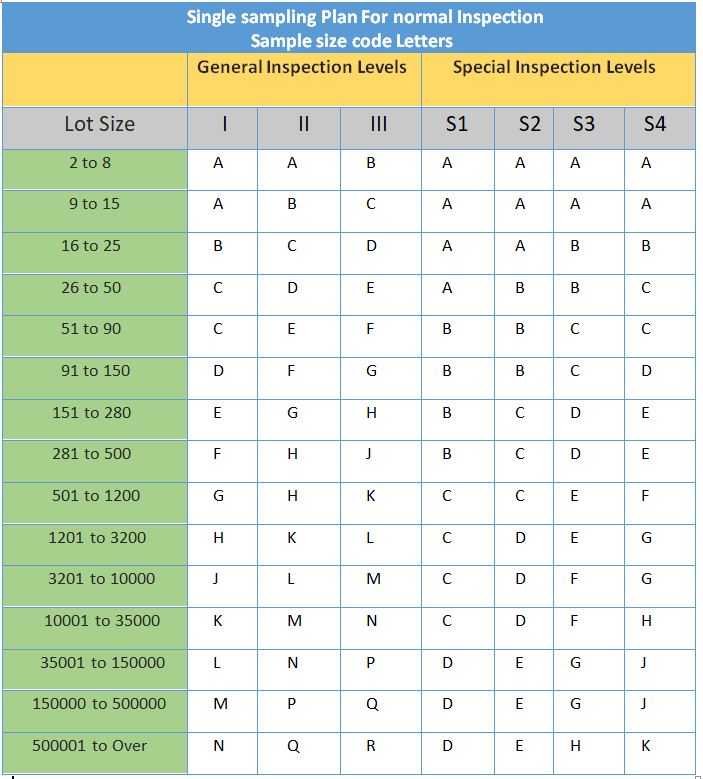
5.2 To determine the code letter corresponding to the lot/ batch size, refer to “the sampling plan and acceptance criteria for a routine inspection”.
5.3 For coated tablets, the size of the lot shall be equal to the lot size of coated tablets per pan.
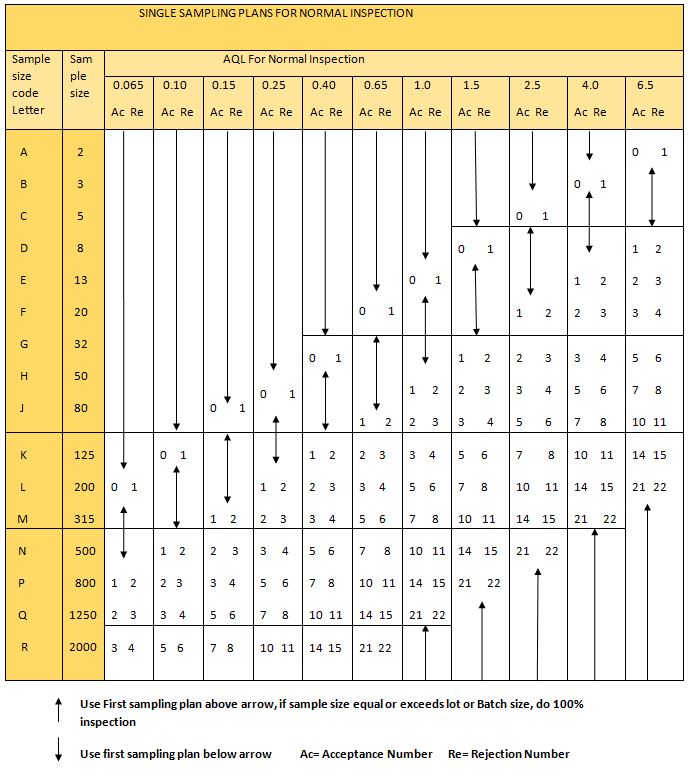
5.4 With the aid of the code letter from step no. 5.2, refer to the sampling plan and acceptance criteria for regular inspection as per the ” sampling plan and acceptance criteria for normal inspection” to determine the sample size.
5.5 To arrive at a representative sample, divide the sample size (step no. 5.4) equally between the total no of containers of batch/lot.
5.6 The concerned QA officer, assisted by the production person, shall collect the sample for AQL.
5.7 AQL shall be conducted on this sample and recorded in the “acceptance quality level for semi-finished (tablets).” which the production handles.
5.8 If the number of unacceptable units found in the sample is equal to or exceeds the rejection criteria, the IPQA officer shall record the same and shall tick 100% inspection in the result column of AQL format.
It shall be checked by section head production and authorized by the section head IPQA (first 100% inspection in case of Uncoated tablets or second 100% inspection in case of coated tablets).
5.9 After 100% inspection of batch/lot, collect the sample as per the sampling plan, and acceptance criteria for tightened inspection follow the” sampling plan and acceptance criteria for tightened inspection” and perform AQL on the same.
5.10 If the number of unacceptable units found in the sample is equal to or exceeds the rejection criteria, the IPQA person shall record the same and shall tick 100% inspection in the result column of AQL format.
It shall be checked by section head production and authorized by section head IPQA (2nd 100% inspection in case of uncoated tablets or 3rd 100% inspection in case of coated tablets).
5.11 After visual inspection of uncoated/ coated tablets, weighing records shall be filled as per “visual inspection uncoated/ coated tablets weighing records.”
5.12 If the number of unacceptable units found in the sample is equal and exceeds the rejection criteria, the IPQA officer shall record the same and tick rejection in the result column of AQL format. Deviation shall rise as per SOP ” Handling of deviation,” and the batch will be segregated for the final disposition by Head QA.
5.13 If any critical defect is observed at any stage of AQL, the IPQA officer shall record the same and tick rejected in the result column of AQL format. Deviation shall be raised as per “Handling of deviation,” and the batch will be segregated for final disposition by the Head QA.
6.0 Acceptance Criteria:
6.1 Each lot/batch should meet the acceptance criteria as per the sampling plan for a routine inspection.
6.2 If the regular inspection fails, then each lot/batch shall meet the acceptance criteria of tightened inspection.
Classification of Defects for AQL in the case of Tablets and Capsules:
Tablets:
Critical defects:
- Embedded specks– partial, which are foreign, extraneous, or contaminant particles to the tablets, the particle is embedded in or on the surface of the tablets, and can not be wiped or blown off.
- Mislabeled- incorrect identification of a product
- Foreign product– Foreign product is the presence of different products other than the desired products.
- Debossed- incorrect debossing.
- Incorrect description– incorrect color/size/shape/ as per specification.
MAJOR DEFECTS:
For Uncoated tablets
- Capped– separation of the lower and upper portion of tablets.
- Broken– complete separation of tablets into substantial parts
- Surface specks– foreign, extraneous particles or contaminants, which can be wiped or blown off.
- Chipped– separation or dislodging
- Mottled– improper distribution of color
- Black particle/oil spots
Read more here: Tablets defects and remedies.
MAJOR DEFECTS:
For coated tablets
- Absence of coating materials in coated tablets
- Incomplete coated materials
- Sticky feel-showing evidence of incomplete drying
- Cracked- the crack in the surface of tablets
MINOR DEFECTS:
It does not affect the use of the product for its indicated purpose but distracts from the appearance of the product, cosmetic.
- Sticked or picked– The irregularity on the upper or lower surface of tablets.
- Picking spots– a small indentation in the surface of the tablet caused by the sticking of the tablets to punch or die.
- Chipped edges– indentation on the edges of tablets.
- Rough edges- tablets exhibit surface imperfections such as spots or blemishes.
- Projected edges– excess edges where the horizontal and vertical parts meet.
- Excessive powder due to malfunctioning of the deduster.
Capsules(Filled):
Major defects :
- Stickiness, cracks, breaks, pinholes, or splits wherever discharge of contents might occur.
- Not a specified (length, or imprint) on the body and cap of capsules.
- Non-uniform in color(s) / look.
- Embedded surface spots or contamination.
- Any foreign matter
- Double shell, loose closure, oversized.
- Bad connexion
- Bubbles
- Wrinkles
- Illegible imprint
- Short capsules
Minor Defects:
- Pits or dents
- Capsule not freed from specks or spots
- Capsules not freed from cap and body cutting into each other.
- Surface not sleek
- Adhering surface spots
- Capsule not freed from powder
- Poor print (broken, heavy, smeared, light, off-center, multiple).
- Ink marks (spots, smears).
Packing Defect for AQL:
Major Defects Packing :
- Incorrect count filled into containers(ii)
- Improper sealing of HDPE containers
- Illegible coding of batch details
- Undesired marks, hair, or spots showing on the first instrumentation on the surface / within the surface
Minor defects
- Adhering surface spots on closures/containers
- Loose/slanted, crimpled/stained label
- Absence of cotton/ desiccant in a container
7.0 Abbreviations:
- SOP- standard operating system.
- IPQA- in-process quality control.
- AQL- acceptance quality level.
- AC – acceptance.
- RE- rejected.
Related Topics:
- SOP on Temperature Mapping in RM, PM, FG, and Control Sample Area
- SOP on initiating, incorporating, approval and recording changes to the master documents
- SOP on Change Control / FDA Change Control
- SOP on collection of Swab and Rinse Water samples
- SOP on Storage and Hold time study for Products
- SOP for Coding and Calibration of Standard Weight

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at Contact@pharmaguddu.com.

