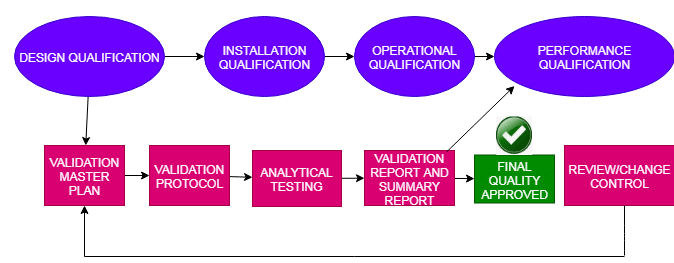
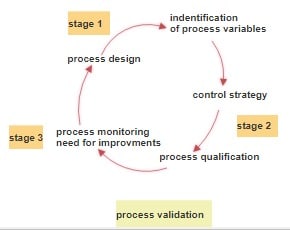
List of Quality Assurance SOPs in Pharmaceuticals
Pharma SOPs is used as a tool for providing a proper and systematic way to follow all rules and regulation under controlled manners in pharmaceutical industries. The quality assurance department reviews all SOPs before the distribution to the department. Read here the list of all Quality Assurance SOPs. List of all SOPs Related to Quality … Read more