Change is driven by innovation, continuous improvement, process performance and product quality monitoring, and CAPA. A firm must have an effective change management system in place in order to effectively review, authorize, and execute these changes. The complexity of change management processes before the original regulatory submission and after submission, where revisions to the regulatory file may be necessary under regional regulations, varies.
Our change management process ensures that continuous improvement is carried out in a timely and efficient way. It should give a high level of certainty that the modification will have no unexpected impacts. Now let’s get into the CMS lifecycle and its applications.
Change Management System Lifecycle:
The following elements should be included in the change management system, depending on the stage of the lifecycle:
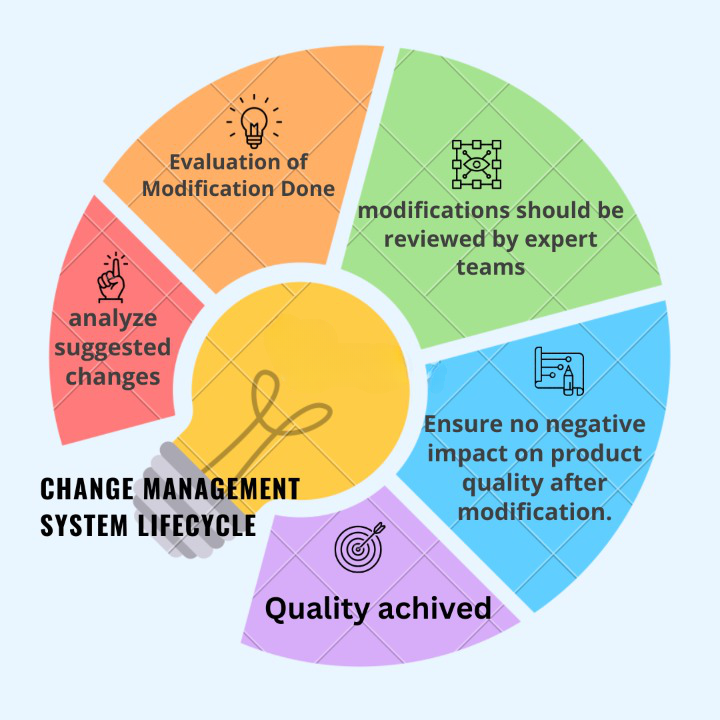
- To analyze suggested changes, quality risk management should be used. The effort and formality of the assessment should be proportionate to the level of risk.
- Proposed modifications should be evaluated in relation to marketing authorization, including design space, when applicable, and/or current product and process understanding.
- An evaluation should be performed to evaluate whether a revision to the regulatory filing is needed under regional regulations.
- Working in the design area, as indicated in ICH Q8, is not considered a change (from a regulatory reporting standpoint). However, from the perspective of a pharmaceutical quality system, all modifications should be examined by a company’s change management system.
- Proposed modifications should be reviewed by expert teams composed of people with relevant skills and knowledge (e.g., Pharmaceutical Development, Manufacturing, Quality, Regulatory Affairs, and Medical) to ensure that the change is technically justifiable.
- A proposed modification should have prospective assessment criteria established.
- Following implementation, the change should be evaluated to ensure that the change objectives were met and that there was no negative impact on product quality.
Related: ICH Guidelines
Application of CMS
Pharmaceutical Development: Change is an integral ingredient of the development process and should be documented; the formality of the change management process should be appropriate for the stage of pharmaceutical development.
Technology Transfer: The CMS should offer management and documentation of process changes performed during technology transfer activities.
Commercial Manufacturing: For commercial production, a formal change management system needs to be in place. The quality control department should give assurance of proper modification and risk-based evaluations.
Product Discontinuation: Any changes made after a product has been discontinued should go via an appropriate change management system.
References:
Food and Drug Administration (FDA)

Panks Pamyal is a Author and Editor at Pharmaguddu.com. He Worked in Top Pharmaceuticals MNCs in India had a more then 10 years experience in Quality control department. He Delivering most valuable insights and knowledge through this website.
