The world is evolving rapidly, with new diseases and disorders emerging, leading to a higher demand for novel discoveries, including new drug findings. There is consistently high demand for aligning medicinal products to uphold and ensure quality, safety, and efficacy standards for each product. The ICH Guidelines for pharmaceuticals play a significant role in ensuring the Quality, Safety, and Efficacy of drug products.

ICH Guidelines Summary/History
ICH stands for International Council for Harmonization. The guidelines focus on technical requirements for pharmaceuticals for human use. Skylights are trying to provide a summarized view of ICH guidelines in 2021 as the ICH updates them annually. These updates will be very helpful for aspiring students and pharmaceutical professionals. Moving on, let’s first explore the history of ICH, an international nonprofit organization that aims to deliver harmonized guidelines for global pharmaceutical development.
- ICH founded: ICH initially founded ICH in April 1990.
- Reason behind ICH founded: ICH helps harmonize technical requirements to ensure product quality, safety, minimize animal testing, and prevent duplication of products and trials.
- Where ICH formed: ICH established its office in Brussels, Belgium. The current ICH Secretariat is located in Geneva, Switzerland.
- Who are the founding members of ICH: The founding members of ICH are the European United States and Japan. The management committee deals with medical terminologies for sharing regulatory information; the ICH secretary is responsible for the day-to-day management of ICH activities.
The coordinator’s members ensure the proper distribution of ICH documents. The ICH working room is established for the selected technical topic for harmonization. There are different types of ICH groups, including the WG and the expert working group. The first group comprises subject matter experts. The second group, the implementation working group, deals with questions and answers. The third group, the informal working group, finalizes the concept papers and moderates the discussion.
The ICH Guidelines Process Steps:
- Step 1: Prepare the conscious draft of the technical document based on the objectives outlined in the concept paper.
- Step 2: EWG confirms the concepts of the technical document and adopts it as a draft Galen by the regulatory members.
- Step 3: This step is further classified into the following:
- First, consultation
- Second, discussion
- Third, finalization process
- Fourth, adoption of an ICH harmonized daily
- Fifth, the final step involves regulatory implementation.
The ICH guidelines in pharmaceutical industries are classified into Quality, Safety, Efficacy, and Multidisciplinary.
List of ICH guidelines:
ICH Quality Guidelines:
The conducting of stability studies, definition of suitable criteria for impurities testing, and a more flexible approach to pharmaceutical quality based on Good Manufacturing Practice (GMP) and risk management are all examples of Harmonisation successes in the Quality field.
Stability guidelines (Q1A to Q1F):
- Q1A(R2): Stability guideline that deals with the stability testing of new drug substances and drug products.
- Q1B: Is about Photo Stability testing of new drug substances and products.
- Q1C: Testing for stability on New dosage form.
- Q1D: Bracketing and Matrixing design for stability testing on new products and substances.
- Q1E: Stability data Evaluation.
- Q1F: Based on climate zone III, and IV, was Removed in June 2006
Guidelines for Analytical validation (Q2):
- Q2 (R1): The analytical validation covers the validation of analytical procedures and text on methodology.
- Q2(R2) /Q14: EWG Analytical procedures evolution and revision of [Q2(R1)] Analytical Validation.
Guidelines for impurities (Q3A-Q3E):
- Q3A(R2): The impurities which discuss their impurities in the new drug substance.
- Q3B(R2): Impurity in new drug products.
- Q3C(R6): Guidelines Dealing with Residual solvent.
- Q3C(R8): Maintenance: for residual solvents.
- Q3D(R1): For elemental impurities.
- Q3D(R2): The revision of Q3D(R1) for transdermal and cutaneous products.
- Q3D: Training: includes in Oct. 2014 on the operation of basic impurities guidelines.
- Q3E: This topic was included in June 2019 by ICH (For judgment and control over extractable and leachable pharmaceuticals and biologicals.
ICH guidelines for Pharmacopoeias (Q4A-Q4B):
- Q4: For Pharmacopoeias as given below:
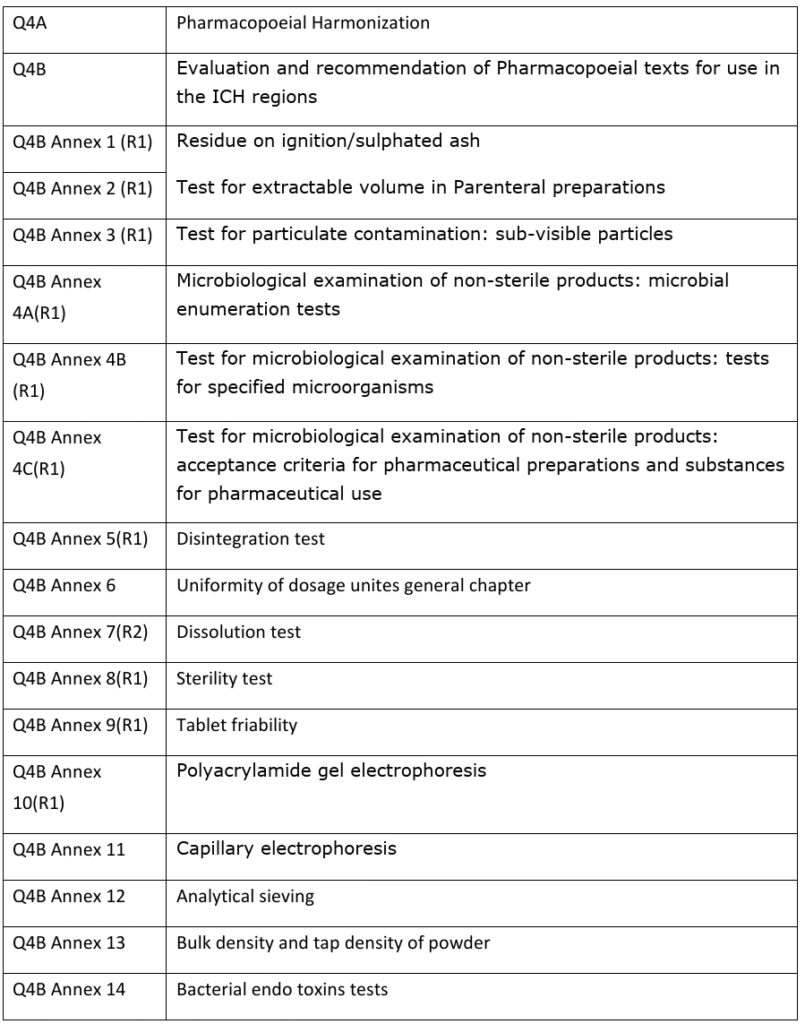
ICH Guidelines On Biotechnological products (Q5A-Q5E):
- Q5A(R1): One is the quality of biotechnical products, which covers the viral safety evaluation of biotech products. Extract from cell lines of humans and animals.
- Q5B: It involves the analysis of cells that are used for r-DNA production.
- Q5C: Dealing with the Quality of biotechnological/ biological products.
- Q5D: Used for biotechnological/biological cell characterization.
- Q5E: Related to the manufacturing process of biotechnological/biological.
Guidelines On Specification (Q6A-Q6B):
- Q6A: used for the testing procedures and their acceptance criteria for new drug products and substances.
- Q6B: For biotechnological/biological test procedure and acceptance criteria.
Others:
Q7: Related to Good Manufacturing Practice.
Q8: Pharmaceutical development.
Q9: Quality risk management provides the principles for quality risk management.
Q10: Develop the Quality system.
Q11: Is the development and manufacture of Drugs, machines that address the development and manufacture of drug products.
Q12: Lifecycle management provides the framework for the product lifecycle.
Q13: This is the continuous manufacturing of Drug substances and drug products, which guides the development and implementation to integrate the continuous manufacture of the products.
Q14: The analytical procedure development is proposed to harness the scientific approaches of the analytical procedure.
ICH Safety guidelines
S1A: Discuss the carcinogenicity studies for neutrals and testings.
S2A: Discuss genotoxicity spacings and studies.
S3A: Discusses toxicokinetic and pharmacokinetics testing and strategies.
S4A: Discuss toxicity testing.
S5A: Discuss reproductive toxicology.
S6A: Discuss biotechnological products that cover preclinical safety requirements.
S7A: Displeased in pharmacological studies.
S8: Discuss criminological studies.
S9: Risk is about the non-clinical evaluation of anti-cancer pharmaceuticals.
S10: Discuss photo safety evaluation which discusses the standards for photo safety.
S11: Discuss non-clinical pediatric safety, which provides the direction of non-clinical safety studies in pediatric development programs.
S12: Discusses non-clinical biodistribution considerations for gene therapy products.
ICH Efficacy guidelines:
E1: Guideline states the clinical safety of drugs in long-term treatment.
E2A-E2F Pharmacovigilance:
- E2A: Study about clinical safety data management.
- E2B(R3): It studies data management, clinical safety, and data elements for transmission ICSRs (individual case safety reports).
- E2C (R2): Studies about periodic benefit-risk evaluation report
- E2D: Data management for post-approval safety.
- E2E: Pharmacovigilance Planning.
- E2F: Update report on development safety.
E3: Guideline to discuss the pinnacle study reports and their clinical study report.
E4: For guideline discusses the dose-response studies and their relationship among drug concentration.
E5: Discuss the ethnic factors that are related to culture, and that could affect the results of clinical studies
E6: Guideline is about good clinical practice and its GCP principles.
E7: Guideline discusses the clinical trials in the geriatric population.
E8: Guideline discuss the general considerations for clinical trials.
E9: About statistical principles for clinical trials.
E10: Guideline discusses the choice of the control group in the clinical trials.
E11: Guideline states about clinical trials in the pediatric population.
E12: Guideline states about clinical evaluation by therapeutic category.
E13: This is not in the picture right now, and it is discussing regulatory compliance.
E14: Discuss the clinical evaluation of QT, which is concerned with the design conduct analysis, and interpretation of clinical studies.
E15: Discuss the definition of pharmacogenomics and pharmacogenetics.
E16: Discusses the qualification of genomic biomarkers.
E17: Discuss the multi-regional clinical trials.
E18: Discuss genomic sampling and management of genomic data.
E19: Discuss the safety data collection.
E20: Discusses the adaptive clinical trials, which discussed the design conduct analysis, and interpretation of adaptive clinical trials.
Multidisciplinary ICH guideline:
M1: Discusses the Medical Dictionary for regulatory activities and terminologies.
M2: Discuss the electronic standards.
M3: Discuss the non-clinical safety studies.
M4: The essential standard technical document recommends assembling all the quality safety and efficacy information in a standard format.
M5: Discusses the data elements and standards for the drug and dictionaries.
M6: Discusses gene therapy.
M7: Discuss the mutagenic impurities.
M8: Discusses the eCTD (electronic technical document); it discusses the critical aspects of technical review and impact assessment of issues arising from ICH use.
M9: Discusses the biopharmaceutical Classification systems based on Biowaivers.
M10: Discuss the analytical method validation.
M11: Discuss the clinical electronics structured harmonized protocol.
M12: Discuss drug interaction studies.
M13: Discuss the equivalence for the immediate release of solid oral dosage forms.
Related: ICH Q10 Guidelines
Conclusion:
The values and benefits of high ICH: it helps in reducing development times and resources. It helps more accessible new drug launches, which also helps to have a recognized global standard. In short, ICH helps bring life-saving treatments to patients faster and helps pharma companies develop standard medicinal products.
Reference: ICH Guidelines official sites

Panks Pamyal is a Author and Editor at Pharmaguddu.com. He Worked in Top Pharmaceuticals MNCs in India had a more then 10 years experience in Quality control department. He Delivering most valuable insights and knowledge through this website.
