Lead is a most undesirable impurity in pharma compounds and comes through the use of sulphuric acid, lead-lined apparatus, and glass bottles used for the storage of chemical materials so, we do a Limit Test for Lead to identify the acceptance level of lead in a sample.
Limit test for lead Principle
The limit test of lead is based on the reaction between lead and diphenyl thiocarbazone (dithizone) in an alkaline solution to form a lead dithionate complex resulting producing a red color. later this red color shall be compared with that of the standard.
Apparatus:
The apparatus required for the Lead limit test follows:
- Analytical Balance
- Glass Rod
- Nessler’s cylinder
- Separating flasks
- Beakers
- Separating flask ring
- Test tubes
The Reagent used in a limit test of Lead
- Reagents like ammonium citrate, potassium cyanide, and hydroxylamine hydrochloride are used to make pH optimum so interference and influence of other impurities have been eliminated.
- The phenol red indicator is used to develop the color at the end of the reaction.
- Lead present as an impurity in the substances gets separated by extracting an alkaline solution with a dithizone extraction solution.
Lead limit test Reaction
Dithizone Reaction: As per IP and USP

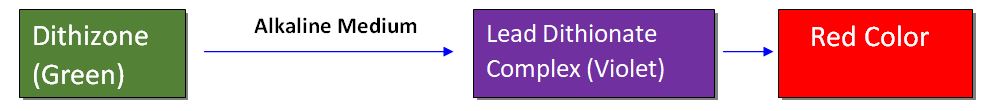
Dithizone is green in color in chloroform and the lead-dithionate complex is violet in color, so the resulting color at the end of the reaction is red.
Permissible limit:
20 ppm is a permissible/ acceptance level limit for lead.
The Procedure to employ for the lead limit test
This method takes two Nessler cylinders one for the test and the other for the Standard test.
| Test | Standard |
|---|---|
| Extract with 5.0 ml of dithizone solution until it becomes green. | Extract with 5.0 ml of dithizone solution until it becomes green. |
| Combine dithizone extract is shaken for 30 minutes with 30 ml of nitric acid and the chloroform layer is discarded. | Combine dithizone extract is shaken for 30 minutes with 30 ml of nitric acid and the chloroform layer is discarded. |
| Add 5 ml of standard dithizone solution to the acid solution. | Add 5 ml of standard dithizone solution to the acid solution. |
| Add 4 ml of CH₄N₂ (ammonium cyanide). | Add 4 ml of CH₄N₂ (ammonium cyanide). |
| Shake well for about 30 minutes. | Shake well for about 30 minutes. |
| Observed the color. | Observed the color. |
Observation:
The intensity of the color complex depends on the amount of lead in the solution. the color produced in the sample solution should not be greater than the standard solution. if the color produced in the sample is less than the standard solution, the sample passes the limit test of lead.
Related: Limit Test for Sulphate/ Limit Test for Chloride/ Limit Test for Iron and Limit Test for Arsenic
Note: PDF and Notes will be added soon so, checkout later.
References:
Indian pharmacopoeia; 2016.
Pharmaceutical Analysis Vol. – I by Dr. A. V. Kasture, Dr. K.R. Mahadik, Dr. S.G. Wadodkar, Dr. H.N. More:
The British Pharmacopoeia.

Panks Pamyal is a Author and Editor at Pharmaguddu.com. He Worked in Top Pharmaceuticals MNCs in India had a more then 10 years experience in Quality control department. He Delivering most valuable insights and knowledge through this website.
