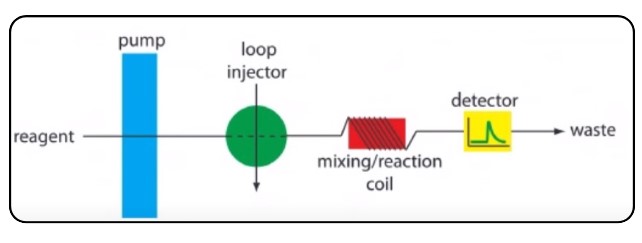
Flow Injection Analysis (FIA) Overview
The instrument being discussed in this context is known as a Flow Injection Analyzer (FIA). Its primary function is to automate several processes typically associated with conventional wet chemistry procedures. Specifically, the Flow Injection Analysis is designed to perform tasks such as automatically picking up samples, mixing them with the required reagents, and subsequently detecting … Read more