In this experiment, we will focus on the preparation and standardization of a 1 M Sodium Hydroxide solution.
Molarity and How to Prepare 1 M NaOH with Example:
- Molarity is defined as the moles of a solute per liter of a solution 1M NaOH solution means 1 Mole of NaOH dissolves in 1 liter CO, free water (solvent)
- 1Mole of NaOH = Molecular weight of NaOH
- The molecular weight of NaOH = The sum of atomic masses of all atoms in a molecule.
Example for factor calculation for standardization of 1 m NaOH:
Na=23
O=16
H=1
Total= 40 gm.
Reagents Required for Preparation and Standardization of 1 M NaOH:
| 1 | Sodium Hydroxide |
| 2 | Potassium Hydrogen Phthalate |
| 3 | Phenolphthalein Solution |
Method for Preparation of 1 M Sodium Hydroxide Solution:
- The method of preparation for the 1 M Sodium Hydroxide solution involves dissolving 42 g of sodium hydroxide in a sufficient amount of carbon dioxide-free distilled water, resulting in a final volume of 1000 ml.
Next, the method of standardization is as follows:
- Accurately weigh about 2.0 g of potassium hydrogen phthalate, which should have been previously powdered and dried at 120°C for 2 hours.
- Dissolve the potassium hydrogen phthalate in 75 ml of carbon dioxide-free distilled water.
- Add 0.1 ml of phenolphthalein solution.
- Titrate the solution with sodium hydroxide until a permanent pink color is obtained.
- Each milliliter of the 1 M sodium hydroxide solution is equivalent to 0.2042 g of C8H5KO4.
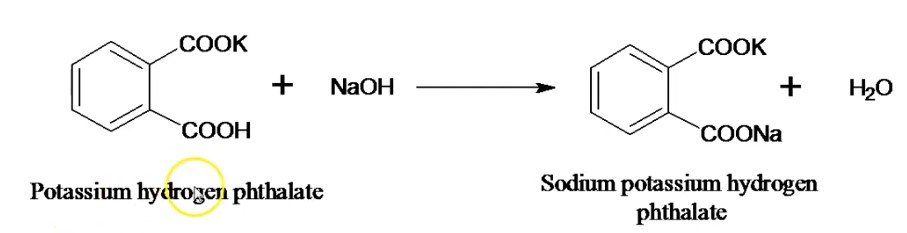
The chemical reaction between potassium hydrogen phthalate+NaOH:
Formula for calculation for the assay is determined as follows:
Assay (%) = (Weight of KHP (g) × 1.0 × Assay of KHP) / (Burette Reading in ml × 0.2042 × 100)
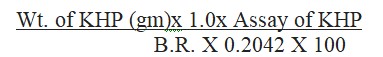
Preparation and standardization of NaOH solution lab report:
To get the final report, Titrate the solution with sodium hydroxide until a permanent pink color is obtained.
This experiment adheres to the guidelines outlined in the “Indian Pharmacopoeia,” ensuring accuracy and reliability in the results.
Related: Preparation and Standardization of 1.0 M Hydrochloric Acid

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
