Name: Preparation and Standardization of 1.0 M Hydrochloric Acid in Pharmaceutical Laboratory.
Reagents:
Use the following reagents to prepare and Standardization of 1.0 M Hydrochloric Acid:
| 1 | Hydrochloric Acid. |
| 2 | Anhydrous Sodium Carbonate |
| 3 | Methyl Red |
Method of Preparation of 1.0 M Hydrochloric Acid:
To make 1.0 M hydrochloric acid, dilute 85.0 ml of concentrated hydrochloric acid with purified water until you have a total volume of 1000 ml.
Related: Preparation and Standardization of 0.1 N HCl
Method of Standardization of 1.0 M Hydrochloric Acid:
- Accurately weigh about 1.5 grams of anhydrous sodium carbonate, which has been heated at around 270°C for 1 hour.
- Dissolve the sodium carbonate in 100 ml of distilled water and then add 0.1 ml of methyl red solution.
- Gradually add the hydrochloric acid by using a burette, while constantly stirring, until the solution turns faintly pink.
- Heat the solution to boiling, let it cool, and then continue the titration.
- Heat the solution to boiling again and continue titrating until the faint pink color is no longer affected by continued boiling.
- Each milliliter of 1.0 M hydrochloric acid is equal to 0.05299 grams of Na2CO3.
Calculation for Preparation and Standardization of 1.0 M Hydrochloric Acid:
Calculate the assay of Na2CO3 using the formula:
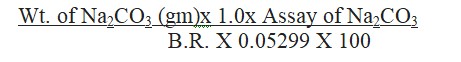
Validity: The prepared 1.0 M Hydrochloric Acid is valid for 1 month from the date of preparation.
Re-Standardisation is required after 15 days from the date of preparation and as needed. If the solution is not used within 15 days, it must be standardized again just before use.
Precaution: Before use, check the physical appearance of the solution. Look for any signs of fungal growth, sedimentation, or changes in color.
Storage: Store the 1.0 M Hydrochloric Acid in glass bottles. Ensure the bottles have well-fitted, suitable stoppers to prevent leakage and contamination.
Reference: Indian Pharmacopoeia

Panks Pamyal is a Author and Editor at Pharmaguddu.com. He Worked in Top Pharmaceuticals MNCs in India had a more then 10 years experience in Quality control department. He Delivering most valuable insights and knowledge through this website.
