1.0 Objective: To lay down a procedure for microbiological monitoring of compressed air used in manufacturing areas.
2.0 Scope: This SOP shall be applicable for a total viable aerobic count of compressed air of “O” Zone and Class 10,000 (liquid manufacturing) area at Manufacturing area.
3.0 Responsibility:
Execution: Tr. Executive and above –QC Department
Checking: Asst. Manager and above–QC Department
4.0 Accountability:
Head of the QC Department
5.0 Procedure:
5.1 Prerequisites :
- S.S. filter holder, 0.45-micron membrane filter, butter paper, airflow meter.
- Soyabean casein digest agar medium
5.2 Microbiological Monitoring of Compressed Air at Point of Use in the manufacturing area.
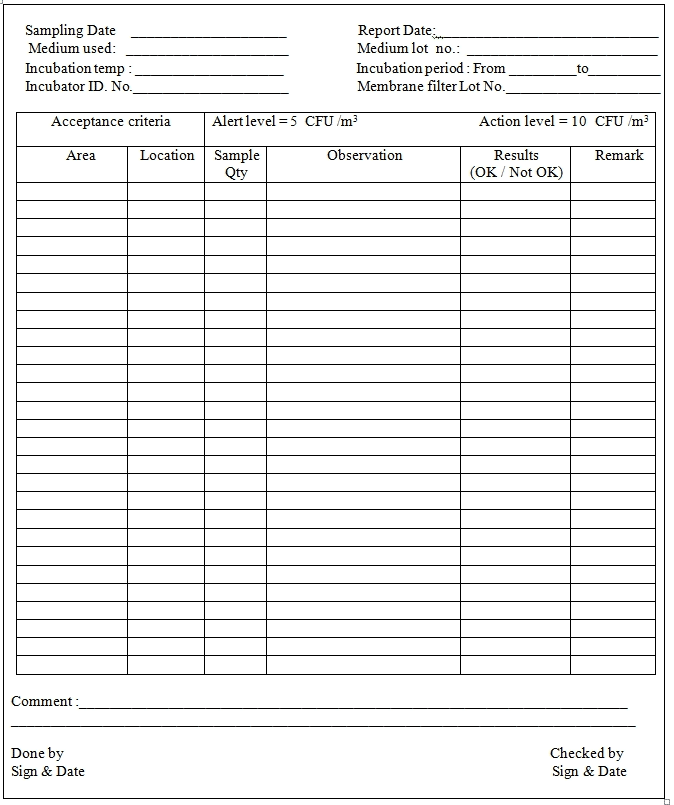
Acceptance Criteria:
Alert level: 5 CFU / m3
Action level: 10 CFU / m3
5.3 Prepare the media plate as per the given SOP.
5.4 Keep the 0.45 µ membrane filter in the S.S. filter holder.
5.5 Wrap filter assembly and required accessories with butter paper and sterilize.
5.6 Carry all accessories into a closed S.S. container to the sampling location to avoid any accidental contamination.
5.7 Connect the filter assembly with the compressed air line with the help of a flow meter to regulate the flow rate of compressed air.
5.8 Allow the flow of compressed air in filter assembly through a 0.45-µ membrane filter and sample the 1 m3 (1000 liter) volume of compressed air.
5.9 After sampling stop the compressed air flow and disconnect the filter holder from the flow meter.
5.10 Aseptically open the filter holder, remove the membrane filter and put it on the solid surface of the SCDA media plate, and write the following details on the plate:

5.11 Repeat the same with all user points to be sampled.
5.12 Incubate the SCDA media plate along with one unexposed plate (Negative control) of the same media lot at 30°C – 35°C for 5 days.
5.13 After following the incubation period count the number of CFU present on the plate.
5.14 Negative control (Unexposed Plate) should not have any growth.
5.15 Record the results in Annexure-I.
5.16 If the results exceed the alert level, allow the production activities and follow the below procedure:
5.16.1 Investigate the matter by reviewing the data available and verifying the accuracy of the test procedure, sampling procedure, and analyst training, if any discrepancies are found, invalid the results and re-confirmed.
5.16.2 Simultaneously inform QA / Engg. Dept. to investigate the root cause by reviewing the data available of operation, maintenance, cleaning, calibration, and performance verification of the compressor to take appropriate corrective and preventive actions, if required.
5.17 If the results exceed the action level, stop the manufacturing activities in the area and follow the below procedure:
5.17.1 Investigate the matter by reviewing the data available and verifying the accuracy of the test procedure, sampling procedure, and analyst training, if any discrepancies are found invalid the results and re-confirmed.
5.17.2 Simultaneously inform QA / Engg. Dept. to investigate the root cause by reviewing the data available on operation, maintenance, cleaning, calibration, and performance verification of the compressor to take appropriate corrective and preventive actions, as required.
5.18 Trend of results:
5.18.1 Identify the colonies present on the plate based on colony characteristics, if any new colony other than routine microflora is observed, isolate and identify the organism as per given SOP to establish the routine microflora information data as per given SOP.
5.18.2 Quarterly prepare a trend of results with reference to alert and action levels in form of a graph and chart.
5.18.3 Annually prepare a review report based on the available trends data.
Related SOP: Procedure for Growth Promotion Test in Microbiology
6.0 Abbreviations
SOP: Standard Operating Procedure
No. : Number
Dept. : Department
CFU: Colony-forming unit
hrs: Hours
SCDA : Soyabean casein digest agar
CFU: Colony-forming units.
m3: Meter cube
NLT: Not less than
NMT: Not more than
µ: micron
S S: Stainless steel
Annexure-1:

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
