1.0 OBJECTIVE: 1.1 Objective of this Standard Operating Procedure is to provide guidelines for the Cleaning and Operation of the Polarimeter. The fundamental principle of polarimetric analysis is based on the existence of optical activity in substances, ie. The ability of a substance to rotate plane-polarized light.
2.0 SCOPE: 2.1 Standard Operating Procedure is applicable for the Cleaning and Operation of Polarimeter in the Quality Control department.

3.0 RESPONSIBILITY
3.1 Preparation: Quality Control chemist
3.2 Review: Head Quality control
3.3 Approval: Head of Quality Assurance Department
4.0 DEFINITION (S)
4.1 SOP: Detailed written instructions for procedures routinely performed in the course of any of the activities associated with pharmaceutical manufacturing. They are used to achieve uniformity of performance.
4.2 Format: An approved designed layout of a document having space for recording/data entry as a part of the documentation.
5.0 PROCEDURE
5.1 Operation
5.1.1 Turn on the mains and wait for the sodium lamp to glow with its full intensity.
5.1.2 Adjust the temperature of the solution whose optical rotation is to be determined to 25°C.
5.1.3 Fill water in a polarimeter tube, clean both ends with a lint-free cloth or tissue paper & Place it inappropriate place.
5.1.4 Remove the bubble if present by tilting the tube upward or downward.
5.1.5 Turn the control wheel till there is no demarcation (equal intensity should be there) on the telescopic field.
5.1.6 Turn the knurled knob to a measurable point.
5.1.7 Record the analyzer reading by seeing through the eyepiece. It should be zero. This point is known as the balance point.
5.1.8 Replace water with the solution and determine its optical rotation.
5.1.9 Calculate the Optical rotation / Specific rotation.
5.1.10 To determine the optical rotation, take readings in the upper eyepiece and record them. Make sure to take three consecutive readings of both the blank and the sample, then calculate the mean value. Subtract the blank reading from the sample reading and use the formula:
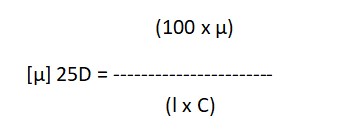
Where:
µ = Observed rotation in degrees at 25°C,
l = Length of the polarimeter tube in decimeters,
C = Concentration of the substance in % w/v,
D = The sodium light line.
5.1.11 Once readings are done, remove the polarimeter tube, clean it, and store it in its box. Finally, switch off the instrument.
5.2 Precautions:
5.2.1 The sample compartment lid should be closed while making a measurement.
5.2.2 The air bubble should be removed before setting zero.
5.2.3 Sample compartment should be cleaned before and after measurement.
5.3 Cleaning :
5.3.1 Remove the electrical connection of the machine.
5.3.2 Open its lid and remove the sample tube from the sample chamber. Open its tip end, purge the sample solution, and wash the tube with Purified water at least three times.
5.3.3 Clean the external surface similarly.
5.3.4 Wash the Tip piece and body tube thoroughly with methanol.
5.3.5 Finally, dry the tube with tissue paper.
5.3.6 Clean the entire working space.
Related: SOP for Magnetic Stirrer in Pharmaceutical
6.0 Abbreviation:
SOP: Standard Operating Procedure
QA: Quality Assurance
QCL: Quality Control Laboratory.
NA: Not Applicable.

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
