1.0 Purpose: To lay down a procedure for Locations, limits, and frequency of nonviable particle count.
2.0 Aim: This SOP is applicable to nonviable particle count locations, limits, and their frequency in the Production facility.
3.0 Responsibility:
3.1 Sr. Officer / Officer & line coordinator shall execute this SOP.
3.2 In-charge / Head shall supervise that the process is being followed as per SOP.
3.3 Head-Quality Assurance shall be responsible for ensuring compliance with this SOP.
4.0 Safety Considerations:
Not Applicable
5.0 Equipment:
5.1 Particle counter
6.0 Procedure:
6.1 Operation of Particle counter:
6.1.1 Take the nonviable particle count as per the “Operation of Particle Counter” SOP.
6.2 Limits of Non-viable particle count:
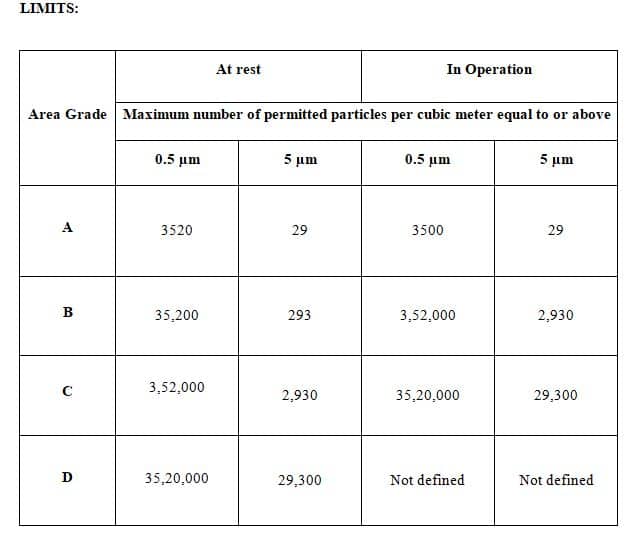
6.2.1 The Limits of non-viable particles according to the respective area are as per Annexure 1.
6.3 Particle Count Locations and Frequency:
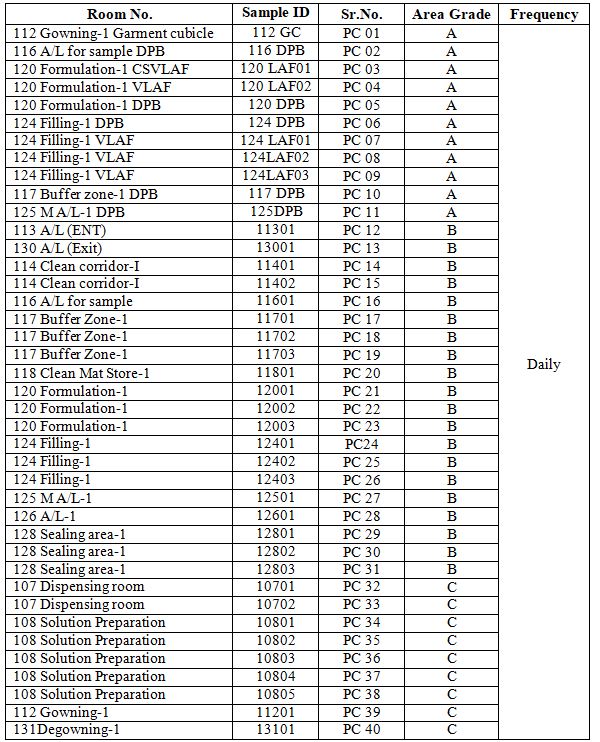
6.3.1 The non-viable particle count locations of the production facility and their frequency of measurement are classified as per Annexure 2.
7.0 Abbreviations: PR: Production
SOP: Standard operating procedure
No. : Number
QA: Quality Assurance
ID: Identification
Cf 3: Cubic feet
HEPA: High-efficiency particulate air
M3: Cubic Meter
Annexure-1
Annexure-2

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].
