ALCOA to ALCOA Plus and Data integrity
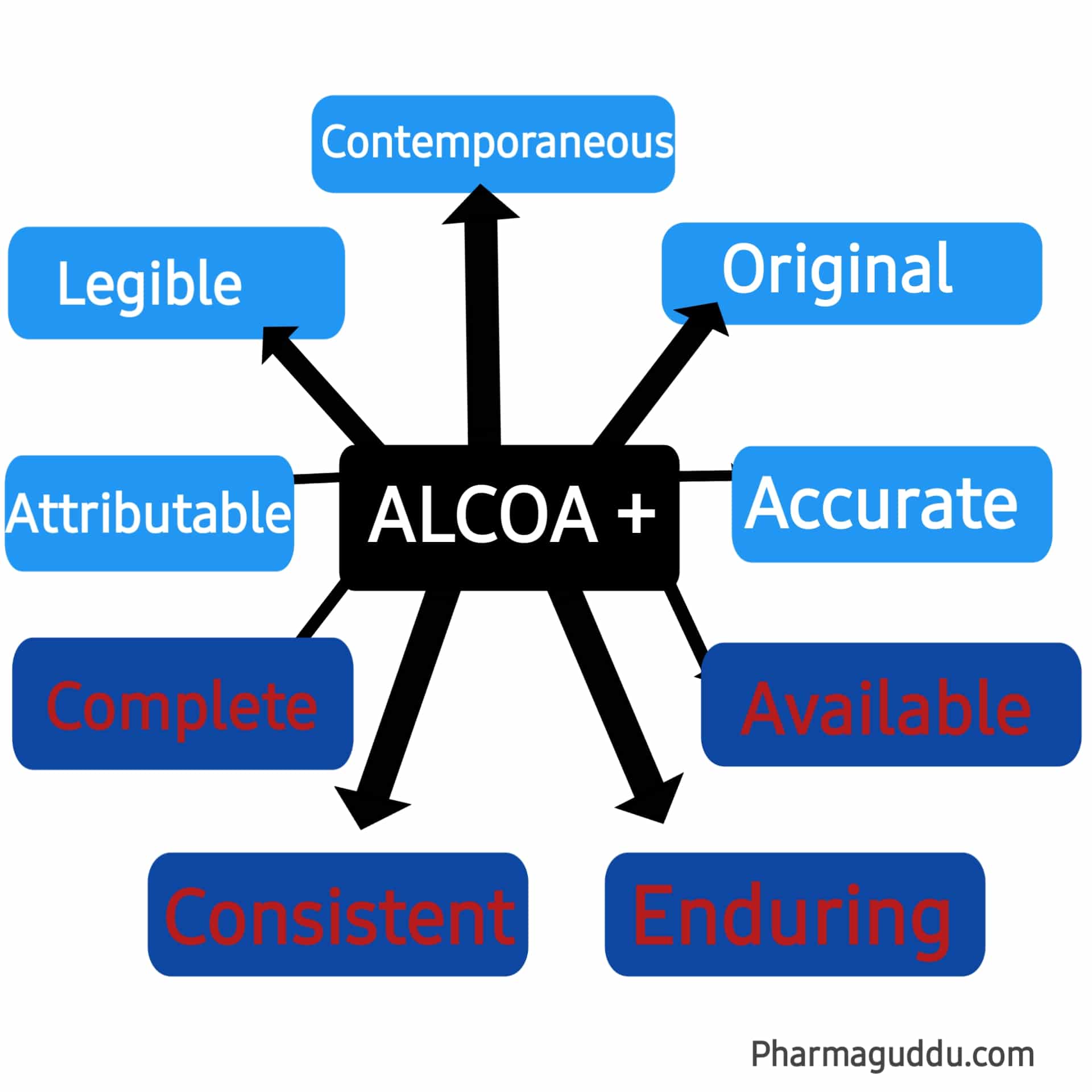
Data integrity is a key approach in the pharmaceutical quality control system. ALCOA stands for (Attributable, Legible, Contemporaneous, Original, and Accurate). It was introduced in the 1990s to ensure the framework for data integrity and good documentation practice (GDP). Then further introduced another term called ALCOA+. ALCO+ stands for (Complete, Consistent, Enduring, and Available) currently used by the … Read more