Water is widely used in pharmaceuticals as an ingredient, solvent for processing, analytical reagent, and in the manufacturing of pharmaceutical products. So, according to pharmacopeia, different types of water are recommended as per the official book and chapter <1231>.
Note: Information in this article doesn’t change the existing guidelines and regulations. It helps users understand pharmaceutical water usage. For more information, read USP chapter <1231>.
The different types of water grades and their use in the manufacturing process are described below.
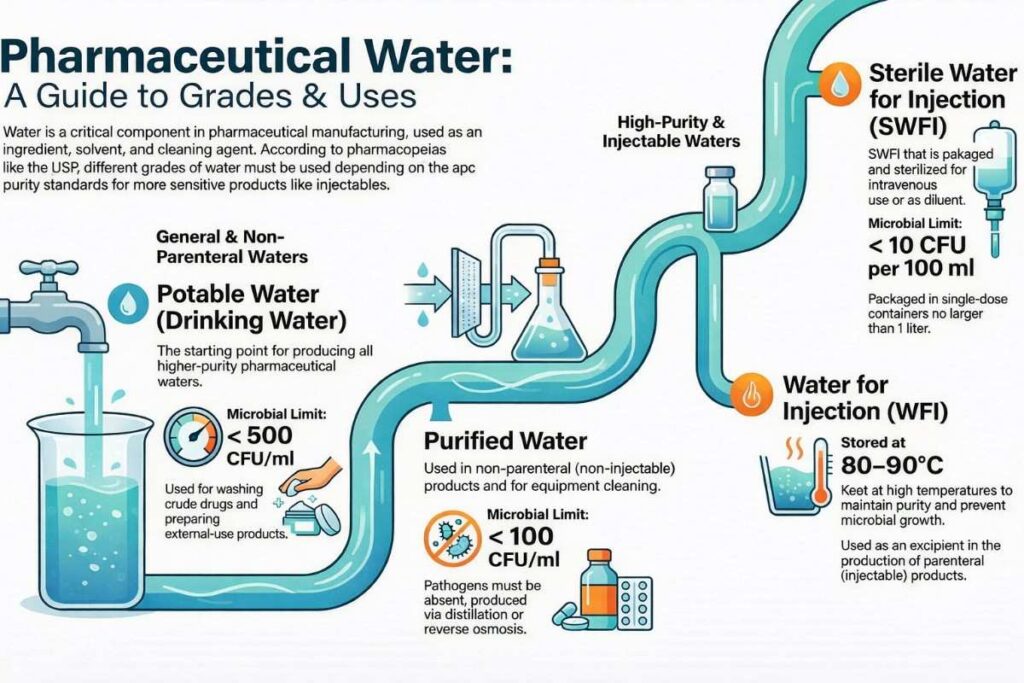
Types of Water in Pharmaceuticals according to USP:
- Drinking or Potable water
- Purified Water
- Water for injection (WFI)
- Sterile Water for Injection (SWFI)
- Bacteriostatic Water for Injection
- Water for hemodialysis
- Sterile Water for irrigation
- Sterile Water for inhalation
- Water for analytical purposes
1. Drinking or Potable water:
Drinking water in pharmaceuticals shall comply with the standards specified by the Bureau of Indian Standards, WHO Drinking Water Standards, and US potable water standard 40 CFR 141. The Indian Pharmacopeia has given a 500 CFU/ml limit for the total aerobic microbial count for drinking potable water.
Purposes of Drinking Water:
- For use as drinking water
- Preparation of external use products
- Washing and extraction of crude drugs
2. Purified Water:
Purified Water means water intended for human consumption and may be sealed in bottles and other containers with no added ingredients.
It is used as a recipient in the production of non-parenteral preparations and for other purposes, such as cleaning certain equipment and non-parenteral products.
Purified water is prepared by distillation, ion exchange, or any other appropriate means from suitable potable water.
The purification process shall comply with standards given in monographs of official books and pharmacopeia. The limit for the microbial population for purified water is 100 CFU/ml. Pathogens shall be absent in purified water.
Difference between Purified Water and Potable Water:
Purified Water: Purified water contains no minerals or additives. It is pure H₂O and only contains hydrogen and oxygen. Purified water has been mechanically filtered to remove all impurities and make it suitable for use. Distilled water is the most common type of purified water.
Purified water is often employed in preparation, drinking, scientific studies, and laboratories. Nowadays, water is frequently purified by processes like capacitive deionization, carbon filtering, reverse osmosis, microfiltration, ultrafiltration, and ultraviolet oxidation.
Potable Water: Potable water is water that is fit for consumption by human beings and other animals. It is also called drinking water. It is safe for drinking and food preparation without the risk of health problems. Water that is contaminated is often treated to turn it into potable water.
One of the easiest ways to treat water is by heating it to a boil. Boiling the water might not remove heavy contaminants, but it can neutralize most bacteria and viruses that may be present.
3. Water for injection (WFI):
Water for Injection (WFI) is a part of the purified water system in pharmaceuticals. WFI is used as an excipient in the production of parenteral products and controls product endotoxins and other impurities when water is used as a vehicle. It is also used to clean parenteral product containers.
The pH of Water for injection ranges from 5.0 to 7.0
Water for injection Usage:
- For the production of parenteral products to protect from microbial growth or contamination.
- For cleaning purposes of contact part components in parenteral products.
Preparation Technique of Water for Injection:
Water for injection is prepared by Distillation of potable Water or Purified Water, Reverse osmosis, and Membrane process.
Storage of Water for Injection (WFI):
The first portion of the distillate is discarded, and the remaining is collected and stored in a special tank containing ultraviolet lamps or in a sealed container. It can be stored for a period of up to a month.
Difference between purified Water and WFI
Water for Injection is highly purified, as it is condensed and kept at 80–90 degrees Celsius to maintain its properties. It is stored in a single dose for IV administration purposes after adding the required amount of solute. In contrast, purified water is kept at room temperature.
4. Sterile water for injection (SWFI):
When combined with a suitable solute, sterile, non-pyrogenic, distilled water is packaged in a single-dose container for intravenous administration. Additionally, it can be used as a diluent dispenser. It contains no additives, including antimicrobials.

It should be packed only in a single-dose container, not larger than 1 liter. It should comply with the standards given in the monograph of official books or pharmacopeias.
The recommended microbial limit is not more than 10 CFU per 100 ml, and pathogens should be absent.
For both WFI and SWFI, the total organic carbon (TOC) as per IP is not more than 0.5 mg/liter. In contrast, the same limit of TOC for water in USP and BP is 500 ppb.
Preparation Technique for SWFI:
Sterile Water for injection is prepared by distillation of Water.
Use of sterile Water for injection:
It is intended mainly for use as a solvent for parenteral preparations, such as powders for injection. It is distributed as dry due to the limited solubility of its solution.
5. Bacteriostatic Water for Injection:
It is sterile water for injection that has one or more suitable antimicrobial preservatives added.
It is used in parenteral products as a diluent, mainly for multi-dose products requiring continuous content withdrawal. It should be packed in containers not larger than 30 ml, either as single-dose or multi-dose.
6. Water for Hemodialysis:
Water for hemodialysis is used as a diluent for hemodialysis concentrate solutions. It is produced from EPA-regulated drinking water, treated purified water to prevent microbiological and chemical contamination. This water is not intended for injection. It shall be stored in an unreactive container to avoid microbial attack.
7. Sterile water for irrigation:

Sterile Water for Irrigation is Water for Injection that is packaged and sterilized in single-dose containers. Sterile Water for Irrigation can be stored in container sizes larger than 1 liter. It allows quick delivery of contents, and it is not mandatory to meet the requirements for small-volume injections.
It is used for applications that do not have particulate specifications or wherever purified water or Water for Injection is indicated, especially when access to a good water system is absent or larger quantities of sterile water are required.
8. Sterile Water for inhalation:
Sterile Water for Inhalation is Water for Injection that is packaged and sterilized. It is employed in inhalators and in the preparation of inhalation solutions.
It is not suitable for parenteral applications because it carries less stringent specifications for bacterial endotoxins than Sterile Water for Injection.
9. Water for analytical purposes:
Distilled water, freshly distilled water, deionized water, high-purity water, and carbon dioxide–free water are incorporated for analytical purposes.
Uses and Benefits of Water in Pharmaceutical:
Water may be present as an excipient, or it may be used to reconstitute products during synthesis, during the production of the finished product, and as a cleaning agent for rinsing equipment, primary packing materials, and vessels.
Conclusion:
This Topics concluded that Water for pharmaceutical management is a critical part of the pharmaceutical manufacturer throughout the operation.
It should also be noted that stainless steel tanks for decalcified and purified Water can be sterilized regularly through clean steam.
Therefore, validation of the system will ensure dependable water production within specified limits. In this case, it will be easier to prove that the cost of purified water will be competitive and, in practice, less expensive than other water-producing systems.
Frequently Asked Questions (FAQ)
Ans: USP Chapter <1231>
Ans: USP non-monographed water includes drinking water, hot purified water, distilled water, deionized water, deionized distilled water, filtered water, ammonia water, oxygen water, lead-free water, deaerated water, LAL reagent water, and hot water.
Ans: Calcium and Magnesium
Ans: The water system should be observed frequently enough to make sure it is under control and continuing to produce water of a good standard.
Ans: Sterile water for Injection

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at [email protected].


