By definition, Weighing balance calibration is a process of verifying the accuracy and precision of a weighing balance used in pharmaceutical manufacturing. It involves comparing the measurement of the balance to a known standard to ensure that it is providing accurate results. In the pharmaceutical industry, we perform monthly calibration on weighing balances to verify their accuracy.

Check the following parameter While performing Weighing balance calibration:
- Accuracy
- Reproducibility
- Eccentricity
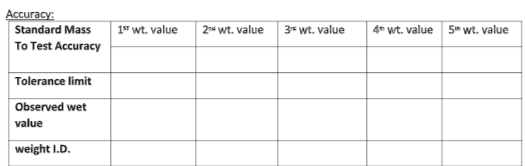
Accuracy:
Verify the balances for accuracy with the minimum weight (least count×100 ),5%, 20%, 50%, and 90% capacity of respective balances. Record the displayed weight in respective monthly calibration formats.
Tolerance limit:
The variation, if any, should be within ± the least count of the balance or ± 0.2% of the certified value of the standard weight used, whichever is higher. For analytical balances, the variation should be within ± the least count of the balance or ± 0.1% of the certified value of the standard weight used, whichever is higher.
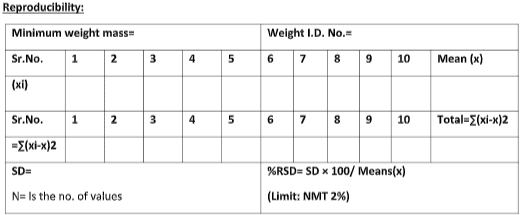
Reproducibility:
To ensure reproducibility, use the minimum standard weight capacity of the balance. Place the weight in the center of the weighing pan and observe the displayed value. Repeat the procedure nine times for the standard weight and record the readings.
Calculate SD and % RSD for both the standard weight by the following formula:
The formula for calculated Standard deviation (SD):

where x= individual value
x̄= mean of the individual value
n= is a number of values.
% RSD= SD*100/ X
Acceptance criteria: %RSD should not be more than 2.0%.
Record the observations in a monthly calibration record as per “monthly calibration record of weighing balance.”
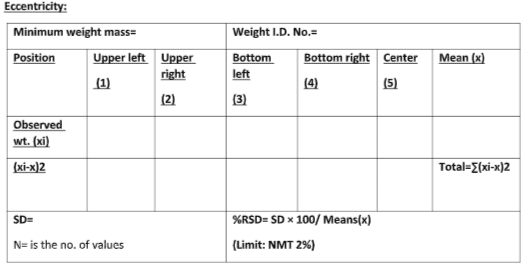
Eccentricity:
Take minimum weight and keep at on specified position as shown below, calculate SD and %RSD by using the formula as specified above.
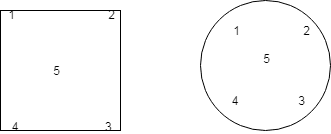
Acceptance criteria:
While calibrating, ensure that the % RSD is not more than 2.0%. Record the calibration details on labels using a marker pen. Write the identification number of the standard weight used during verification and calibration in the designated place of the verification and calibration records. A quality assurance personnel should verify the calibration record by affixing a “Reviewed by Quality Assurance” stamp along with initials and the date. Calibration of the analytical balance in the quality control department should be conducted by their respective SOP.
Related: SOP on Checking Balance Eccentric Accuracy
Calibration of balances by external agency:
Internal calibration (software calibration) of weighing balances shall be done by external agencies. A calibration certificate received from an external agency shall be reviewed including the details of balance and calibration summary.
Action to be taken if out of calibration:
If the Balance is out of calibration, refer to SOP “handling of out of calibration” and raise deviation as per SOP “handling of deviation,” and fix the label “out of calibration” as per SOP and inform to Department Head for Investigation.
Raise a maintenance memo and inform the engineering department to take corrective action. If necessary, obtain a balance of configuration from another section and department to conduct the weighing activity.
In such situations, perform daily balance verification of the transferred balance and utilize it for weighing. If the daily verification parameter does not meet the specifications, conduct monthly calibration. After rectifying the balance, calibrate it and document the results in the respective calibration records. The balance should be released for further use only upon satisfactory closure of the deviation, accompanied by a detailed investigation result.
FAQs on Weighing Balance Calibration:
Ans: Maintain variations within ± the least count or ± 0.2% of the certified weight value.
Ans: Use minimum standard weight, repeat the procedure nine times, and calculate % RSD.
Ans: % RSD should not exceed 2.0%, ensuring accurate and reliable calibration results.
Ans: The least count of a pharmaceutical weighing balance refers to the smallest value that can be measured or displayed by the balance. For Example, if a balance has a Least count of 0.1 mg, it means that the smallest weight that can be measured is 0.1 mg.

Naresh Bhakar is the Founder and Author at Pharmaguddu.com, bringing his extensive expertise in the field of pharmaceuticals to readers worldwide. He has experience in Pharma manufacturing and has worked with top Pharmaceuticals. He has rich knowledge and provides valuable insights and data through his articles and content on Pharmaguddu.com. For further inquiries or collaborations, please don’t hesitate to reach out via email at Contact@pharmaguddu.com.
