There is a provision of guidelines for storage, sales, precriptions and classififcation of each drugs Under the differernt schedules of Drugs and Cosmetic rules 1945. Below is the List of Drugs under Schedule C, C1, G, H, H1, K, P, M, N and X with CAUTION and WARNING.
Drugs SCHEDULE ‘C’
- List of biological and special products (injectable) used with special guidelines.
- Examples include serums, vaccines, antigens, insulin, sterilized surgical threads, disposable medical tools for single use, injectable antibiotics, and more.
- Import is not allowed after the expiration of effectiveness.
- Labeled with the caution: “This preparation should only be used under medical supervision; it can be harmful otherwise.”

SCHEDULE ‘C(1)’
- List of biological and special products (non-injectable) with specific regulations.
- Examples are medicines from the Digitalis group and their preparations, Ergot and its related drugs, vitamins and their preparations, fish liver oil, adrenaline, liver extracts, hormone vaccines, and in-vitro devices for HIV and HCV.
- Import is not allowed after the expiration of effectiveness.
- Labeled with the caution: “This preparation should only be used under medical supervision; it can be harmful otherwise.”
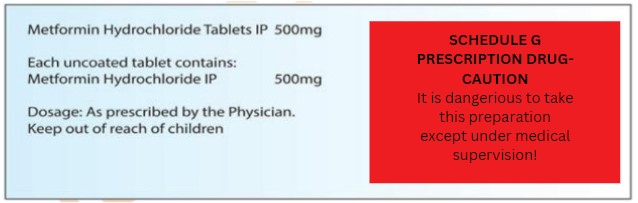
Drugs SCHEDULE ‘G’
- List of substances that require use under medical supervision, with appropriate labels.
- Labeled with the caution: “This preparation should only be used under medical supervision; this label should be clearly visible and not have any other text around it.”
- It’s important to create a proper sales invoice.
- Maintain records of purchasing and selling these medicines for a period of two years.
- Examples include L-asperginase, Bleomycin, Busulphan, Chlorambucil, and metformin, among others.
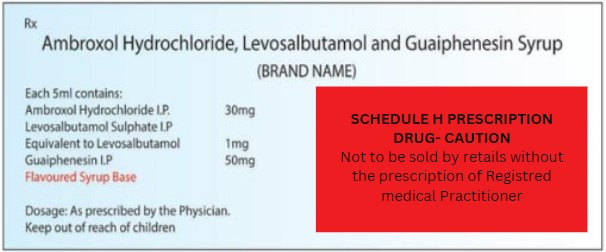
Drugs “SCHEDULE ‘H’
- Schedule H (Drugs & Cosmetics (2nd Amendment) Rules 2006)
- Medicines that need a doctor’s prescription to be bought from a store
- Medicines under Schedule H must have ‘Rx’ symbol on the label’s top left corner.
- Medicines listed in Schedule H, and covered by the Narcotic Drugs and Psychotropic Substances Act, 1985, should have the ‘NRx’ symbol in red on the top left corner of the label.
- Examples: Alprazolam, Allopurinol, Amikacin, Atenolol, Acyclovir, Buspirone, Azathioprine, Captopril, Carbidopa, Clindamycin, Cimetidine, Ciprofloxacin, Cefuroxime, Diclofenac, Glimepiride, diazepam, etc.
Related Topic: History of the Pharmacy Profession
SCHEDULE ‘H1’
- To monitor the use and misuse of antibiotics
- Schedule H of the Drugs and Cosmetics Act contains a list of 536 drugs that require a prescription from a registered medical practitioner.
- To regulate the unauthorized sale of antibiotics separately, a new schedule, Schedule H1, was added in the Drugs and Cosmetics (4th Amendment) Rules 2013.
- Under Schedule H1, drug formulations must be labeled with the ‘Rx’ symbol in red on the top left corner of the label, along with the following words in a box with a red border:
- Warning: It is risky to use this medicine without a doctor’s advice. It cannot be sold in stores without an RMP’s prescription.

Example as below:
| Drugs under schedule H are omitted | Drugs under Schedule H1 |
|---|---|
| Alprazolam | Alprazolam |
| Thiacetazone | Diazepam |
| Cefdinir | Cefdinir |
| zolpidem | Codein |
| Cefepime | Cefepime |
| Ethambutol HCI | Cycloserine |
| Clofazamine | Cefixime |
| Meropenem | Ertapenem |
| Codein | Cefoperazone |
| Ethionamide | Doripenem |
| Diazepam | Ethambutol |
| Imipenem | Nitrazepam HCI |
| Cefpirone | Midazolam Gemifloxacin |
| Pentazocine | |
| Midazolam | Cefpirome |
| Sparfloxacine | Rifampicin |
| Tramadol | Ceftriaxone |
| Chlordaizepoxide | Tramadol |
Drugs SCHEDULE ‘K’
- List of medicines that are not subject to certain rules for making and selling medicines.
- A group of medicines not meant for healing, with each container clearly labeled ‘NOT FOR HEALING’.
- Medicines that still fall under the category of ‘home remedies’ include Paracetamol tablets, pain-relief balms, antacids, calcium products with or without Vitamin D, Gripe Water for infants, Inhalers (with medicines for treating cold and stuffy nose), syrups, lozenges, pills, and tablets for cough, cold, or a sore throat.
- However, these medicines should not contain any substances listed in Schedules G, H, or X of the D&C Act and Rules.
- Shopkeepers must also make sure that they are selling medicines in the original, unopened containers from licensed manufacturers.
- Note: Aspirin and Quinine Sulphate are no longer part of this schedule.”
Drugs Schedule ‘M’
Schedule ‘M’ focus on the requirements for manufacturing facilities, maintaining hygiene and quality. It covers the following areas:
General Requirements: Ensure the overall facility meets necessary standards.
Location and Surroundings: The site should be away from open sewage, public restrooms, excessive dust, smoke, and unpleasant odors, and should not emit harmful chemicals or biological substances.
Buildings and Premises: The design of the buildings should be suitable for manufacturing operations and should be easy to keep clean.
Water System: A validated water treatment system should be in place to make the water safe for use and free from harmful microorganisms.
Waste Disposal: Disposal of waste materials should follow guidelines set by the Environment Pollution Control Board.
Warehousing Area: The design should allow for organized storage of various materials and products.
Production Area: The production area should be designed for efficient and contamination-free operations.
Ancillary Area: Rest and refreshment rooms should be separate, with facilities for changing and storing clothes. Washrooms should be easily accessible.
Quality Control Area: There should be a separate laboratory for quality control, independent of the production area. Adequate space should be provided for different types of testing. All operations should be supervised by qualified technical staff approved by the Licensing Authority.
Documentation and Record-Keeping: All documentation and records should be well-organized, specifying the title, nature, and purpose.
Others subtypes of schdule ‘M’ as follow:
Schedule ‘M1’ – Requirements for Homeopathic Medicine Manufacturing Facilities
Schedule ‘M2’ – Requirements for Cosmetics Manufacturing Facilities
Schedule ‘M3’ – Requirements for Medical Device Manufacturing Facilities
Drugs SCHEDULE ‘N’
- A list of the basic equipment needed to run a pharmacy effectively.
- The entrance should have a sign that says “Pharmacy” in the front.
- The pharmacy area should be separate from private rooms. It should be well-constructed, dry, with good ventilation, and spacious enough to store the products separately.
Drugs SCHEDULE ‘P’
- The lifespan of a medicine.
- The time in months (unless stated otherwise) between the manufacturing date and the expiration date. This is the maximum time the medicine can remain potent under the specified storage conditions.
- This schedule includes antibiotics, vitamins, insulin preparations, normal human plasma, serotoxins, toxoids, other toxins, antitoxins, and various medicines.
| Name of Drugs | Periods in Month | Storage condition |
|---|---|---|
| Adriamycin | 30 | In a cool place |
| Ampicillin Na | 36 | In cool place |
| Carbenicillin Sodium Powder | 24 | At temperature not exceeding 5°C |
SCHEDULE ‘P-1’
- The package size for specific medicines.
- It lists the names of medicines, their forms, and the allowed package sizes.
- Medicines can only be sold in the listed package sizes.
- Some examples of medicines in this schedule:
| Name of Drugs | Dosage form | Pack Size |
|---|---|---|
| ALbendazole | Suspension | 10 ml |
| Atenolol | Tablets | 14 Tablets |
| Piperazine | Granules | 5 gm |
Drugs SCHEDULE ‘X’
- A list of drugs that can lead to addiction or have psychological effects.
- Schedule X includes drugs that:
→ Require careful handling, and pharmacists must ensure they are not sold without a prescription.
→ Have a warning label that says ‘Schedule X drug.’ The warning is: “To be sold at retail only with a prescription from a registered medical practitioner.” The label also has a red ‘XRx’ symbol displayed prominently in the top left corner.
- After selling the drug, the pharmacist must stamp and keep the prescription.
- Keep records of the purchase and sale of the drug and store them for two years from the transaction date.

Some examples of drugs in schedule X:
- Amobarbital
- Amphetamines
- Dexamphetamines
- Glutethimide
- Methylphenidate, and more.

