In this experiment, we will focus on the preparation and standardization of a 1 M Sodium Hydroxide solution.
Molarity and How to Prepare 1 M NaOH with Example:
- Molarity is defined as the moles of a solute per liter of a solution 1M NaOH solution means 1 Mole of NaOH dissolves in 1 liter CO, free water (solvent)
- 1Mole of NaOH = Molecular weight of NaOH
- The molecular weight of NaOH = The sum of atomic masses of all atoms in a molecule.
Example for factor calculation for standardization of 1 m NaOH:
Na=23
O=16
H=1
Total= 40 gm.
Reagents Required for Preparation and Standardization of 1 M NaOH:
| 1 | Sodium Hydroxide |
| 2 | Potassium Hydrogen Phthalate |
| 3 | Phenolphthalein Solution |
Method for Preparation of 1 M Sodium Hydroxide Solution:
- The method of preparation for the 1 M Sodium Hydroxide solution involves dissolving 42 g of sodium hydroxide in a sufficient amount of carbon dioxide-free distilled water, resulting in a final volume of 1000 ml.
Next, the method of standardization is as follows:
- Accurately weigh about 2.0 g of potassium hydrogen phthalate, which should have been previously powdered and dried at 120°C for 2 hours.
- Dissolve the potassium hydrogen phthalate in 75 ml of carbon dioxide-free distilled water.
- Add 0.1 ml of phenolphthalein solution.
- Titrate the solution with sodium hydroxide until a permanent pink color is obtained.
- Each milliliter of the 1 M sodium hydroxide solution is equivalent to 0.2042 g of C8H5KO4.
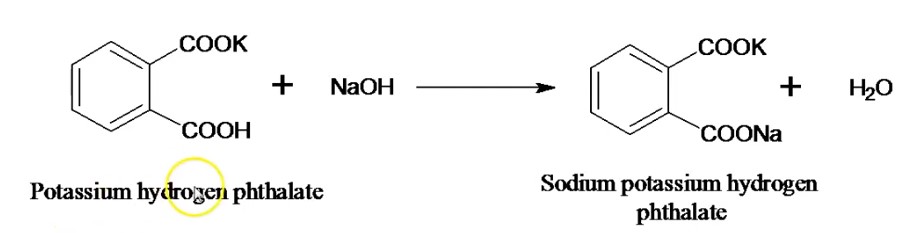
The chemical reaction between potassium hydrogen phthalate+NaOH:
Formula for calculation for the assay is determined as follows:
Assay (%) = (Weight of KHP (g) × 1.0 × Assay of KHP) / (Burette Reading in ml × 0.2042 × 100)
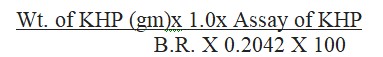
Preparation and standardization of NaOH solution lab report:
To get the final report, Titrate the solution with sodium hydroxide until a permanent pink color is obtained.
This experiment adheres to the guidelines outlined in the “Indian Pharmacopoeia,” ensuring accuracy and reliability in the results.
Related: Preparation and Standardization of 1.0 M Hydrochloric Acid

